 Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order. Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
 Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!  Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!
> Insights > Summary of classic components and pathways in the tumor microenvironment The tumor microenvironment (TME) is comprised of the tumor, extracellular matrix (ECM) and various non-transformed cells including fibroblasts, immune infiltrates, and vascular vessels recruited from nearby local or distant tissues. TME plays a key role in tumor initiation, progression, invasion, metastasis, and resistance by deploying matrices, cytokines, growth factors, as well as vascular networks for nutrient and waste exchange (1). Understanding TME requires a close examination of signaling pathways responsible for its characteristics.
Click to find out more about classic TME signaling pathways
The Hedgehog, Notch and Wnt etc. are groups of evolutionarily highly conserved signaling pathways that determine cellular fate. These pathways are found to be upregulated in TME including cancer stem cells (1) and serve as important targets in cancer therapies(2).
>> Hedgehog Pathway
The canonical Hedgehog (HH) pathway involves autocatalysis of HHC, addition of cholesterol molecule to the N-terminus and secretion of HHN ligand. The binding of HHN to its cognate receptor Patched (PTCH1) releases its inhibitory effect on Smoothened (SMO) resulting in the downstream release of GLI transcription factors. The HH pathway plays a critical role in embryonic development and adult tissue maintenance and regeneration. Hyperactive HH signaling, either mutation in PTCH, SMO or overexpressing of HH, is responsible for basal cell carcinoma, pancreatic, lung, prostate, ovarian and breast cancer. In TME, upregulated HH signaling in tumor cells promotes the growth and survival of neighboring tumor cells via autocrine signaling. While HH ligand produced by epithelial cells can signal mesenchymal/stromal cells which signal back to epithelial cells promoting proliferation and survival in a paracrine signaling manner(2).
>>> Click to find Hedgehog- related products
>> Notch Pathway
The Notch signaling pathway is a juxtacrine (contact-dependent) signaling pathway that mediates cell fate decisions between neighboring cells and is involved in every component of the TME as well as in the interaction between different parts of the TME(3). The Notch receptor is a transcription factor anchored at the membrane and is released following interaction with a cognate ligand. Binding of the Notch ligand leads to the unfolding of the Notch regulatory region (NRR) and unmasking of the cleavage sites for ADAM10/17 and the γ-secretase. Upon cleavage at NPR, the Notch intracellular domain (NICD) is released into the cytosol. The dynamic and strength of NICD production depend on the Notch ligands. These ligands are transmembrane proteins of the Delta/Serrate/LAG-2 (DSL) family. In mammals, this family includes three delta-like ligands (Dll1, Dll3, and Dll4) and two jagged ligands (Jag1 and Jag2) (3).
Notch receptors and ligands are widely expressed in the various compartments of the TME. Researchers have identified that Notch signaling is involved in angiogenesis, activation of fibroblasts, maintenance of the cancer stem cell niche,shape the immune infiltrate at the tumor site and may also be regulated by physical and chemical heterogeneity in the TME (3).
>>> Click to find Notch-related products
>> Wnt
The Wnt signaling pathway is an important regulator of embryogenesis development, tissue homeostasis, stemness control, wound repair, and malignancy. Dysregulated Wnt signaling plays a central role in initiation, progression, and metastasis in many types of human cancers.
In general, there are three pathways: the canonical Wnt pathway, the non-canonical Wnt/planar cell polarity (PCP) pathway, and the Non-canonical Wnt-calcium pathway.
In the canonical Wnt pathway, the Wnt ligand binds to the Frizzled (FZD) receptor, which is a G protein-coupled receptor (GPCR) with seven transmembrane domains and the co-receptor low density lipoprotein receptor-related protein 5/6(LRP5/6). Consequently, β-catenin accumulates and translocates to the nucleus where it can bind to the transcription factor T-cell factor/lymphoid enhancer factor (TCF/LEF) and recruit the transcriptional Kat3 co-activator p300 and/or CREB-binding protein (CBP) to activate downstream Wnt target genes. The genes activated by Wnt have important functions for many processes in oncogenesis and development, such as self-renewal, differentiation, proliferation, and metastasis. Whereas Non-canonical Wnt signaling contributes to the stabilization of proteins other than β-catenin to maintain intracellular functions through these alternative pathways (4).
>>> Click to find Wnt-related products
>> TGF-β pathway
Transforming growth factor beta (TGF-β) is a pleiotropic growth factor. The TGF-β signaling is a conserved pathway that emerged with multicellular organisms and plays a key role in many developmental and cellular processes: TGF-β binds to a single TβRII receptor which brings together two TβRI and two TβRII receptors into a hetero-tetrameric complex. This ligand-mediated assembly triggers the phosphorylation and activation of TβRI by TβRII initiating downstream signaling pathways through their intracellular serine/threonine kinase domains. The signal is transduced through either canonical (SMAD-dependent) or non-canonical (SMAD-independent) signaling pathways (5, 6).
TGF-β signaling pathway is one of the most important signaling pathways in TME, which plays dual roles in carcinogenesis. In early phases, TGF-β functions as a tumor suppressor that blocks tumor growth by inhibiting cell cycle progression in cancer cells and stimulating apoptosis in pre-malignant cells. However, tumor-suppressive effects of TGF-β are often lost in advanced tumors as its tumor pro-invasive functions prevail. The subsequently increased secretion of TGFβ in the tumor microenvironment affects cancer cell growth and stimulates migration, invasion, and angiogenesis, In addition, it is suggested that TGF-β might take a key role in shaping the CAF landscape, especially in light of its connection to myofibroblast differentiation and function in tumors and in other fibrotic diseases(5-7).
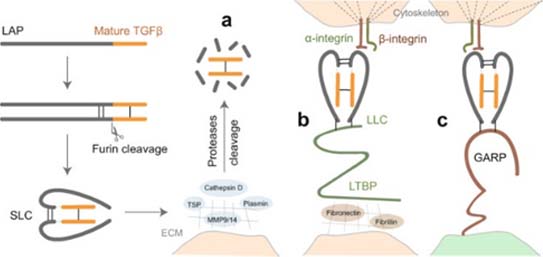
Activation of latent TGF-β signaling begins first with cleavage of its proprotein into the latency-associated peptide (LAP) and the mature TGF-β. The LAP dimer then binds to mature TGF-β forming small latent complex (SLC). There are three major mechanisms for activation of this latent TGF-β.
a) Proteases in the extracellular matrix (ECM) cleavage of LAP and release of active TGF-β. Alternatively, thrombospondin (TSP) can also induce activation of TGF-β by direct binding to LAP.
b) The SLC can be anchored to the ECM proteins via latent TGF-β-binding protein (LTBP) and forms the so-called large latent complex (LLC) which will release active TGF-β by cell contraction after interaction between LAP and integrins.
c) SLC binds to glycoprotein A repetition predominant protein (GARP) on the cell surface and upon interaction with integrin release active TGF-β (6). Several cancer therapeutics targeting TGF-β comprising of small molecules, antisense oligos, antibodies and ligand traps are being explored in clinical trials.
>>> Click to find TGF-β-related products
>> Integrins
Integrins belong to the family of cell adhesion receptors that control cell adhesion to ECM and were comprise of α and β heterodimeric subunits with 24 distinct heterodimers identified to date. Integrins also plays a key role in cell signaling regulating cell proliferation, survival, migration, and invasion. Additionally, Integrin signaling is linked with other receptor tyrosine kinase (RTK) signaling as well as a mediator of downstream signal transduction including PI3-Akt, MAPK/Erk, SAPKs and JNK pathways. In TME, Integrin supports tumor cell adhesion to ECM, angiogenesis and plays a major role in cancer cell metastasis and aggressiveness. Therefore, Integrins are being targeted for anticancer therapy by integrin blockers. Two candidates, an Arg-Gly-Asp (RGD) pentapeptide targeting avb3 and avb5 integrins and volociximab, IgG4 inhibitor of a5b1 are in phase II and III clinical trials(8).
>>> Click to find Integrins -related products
>> RTKs
Receptor Tyrosine Kinases (RTKs) are class of cell surface transmembrane receptors that form cross-linked dimers upon ligand binding leading to activation of its tyrosine kinase activity via cross-phosphorylation followed by signal transduction. RTKs bind to growth factors, hormones and cytokines to elicit a variety of major cellular processes and also play key roles in TME.
>> EGF
Endothelial growth factor receptor (EGFR) is expressed in almost all non-neoplastic cell types, except in the mature cells of the lymph hemopoietic system. Notably, active EGFR signaling in the non-neoplastic cells of the TME is speculated to take a supportive role in tumor cell proliferation, angiogenesis and metastasis. Activation of EGFR is triggered by ligand binding and receptor dimerization followed by cross phosphorylation occurring via Src homology 2 (SH2) and phosphotyrosine binding (PTB) domains. The downstream signaling pathway activated by EGFR is the KRAS-BRAF-MEK-ERK pathway, PI3K pathway as well as AKT and STAT pathways among others.
In the TME, EGFR signaling stimulates the synthesis and secretion of a number of angiogenic regulating factors, such as vascular endothelial growth factor (VEGF), Interleukin-8 (IL-8) and basic fibroblast growth factor (bFGF). Additionally, EGF and tumor growth factor alpha (TGF-α) serve as a potent pro-angiogenic factor that regulates tumor stimulated angiogenesis(9). The main Ras/PI3K pathway downstream signaling upon EGFR activation leads to aberrant expression of VEGF in the TME. Variety of therapeutics target the EGF pathway and EGF-induced production of VEGF including kinase inhibitors gefitinib, PD15035 and monoclonal antibody cetuximab(9).
>>> Click to find EGF -related products
>> FGF
Fibroblast growth factor receptors (FGFRs) are RTKs involved in several biological processes including regulation of tissue development and repair. Alterations in FGFRs 1–4, such as amplification, fusions and mutations, as well as aberrant epigenetic or transcriptional regulation and changes in tumor-stromal interactions in the tumor microenvironment, can lead to the development and/or progression of cancer (10).
During carcinogenesis, FGF triggers the autophosphorylation of FGFR at a key tyrosine residue in an activation loop of the tyrosine kinase domain. FGFR autophosphorylation results in a structural change of the tyrosine kinase domain from an inactive form to an active form. Subsequently, FGF signals are transduced to the RAS-MAPK, and PI3K-AKT signaling branches via FGFRs and FRS2. FGF signals are also transduced to the DAG-PKC, and IP3-Ca2+-releasing signaling branches via FGFRs and cell growth factor PLCγ. FGF signals are involved in stemness, proliferation, anti-apoptosis, drug resistance, angiogenesis, epithelial-to-mesenchymal transition (EMT), and invasion in target cells(11).
>>> Click to find FGF -related products
>> HGF
Hepatocyte growth factor (HGF), also known as scatter factor, is generally secreted by stromal cells and is the native peptide ligand of HGFR/c-MET receptor. The aberrant activation of HGFR/cMET plays important role in the development and progression of several human cancers including lung, renal, gastrointestinal, thyroid and breast carcinomas, as well as sarcomas and malignancies of the nervous system such as glioblastoma multiforme (GBM) among others.
The HGF/MET axis mediates the activation of several downstream signaling such as RAS/RAF/MEK/ERK, PI3K/Akt/mTOR, and JAK/STAT and Wnt/β-catenin pathways. HGF/MET regulates multiple biological processes in cancer cells such as cell proliferation, survival, inhibition of apoptosis, migration, invasion, metastasis and drug resistance(12).
>>> Click to find HGF -related products
>> Summary
The TME is a complex environment of variety of signaling molecules, chemokine, soluble factors and ECM that supports growth, survival and invasion of tumor cells(2). This environment is fostered by stromal cells which surrounds the tumor composed of epithelial cell promoting tumor angiogenesis, fibroblast releasing chemokines, immune cells and inflammatory cells. This network between the stroma and tumor cell is critical and understanding and targeting these signaling pathways will enable development of effective cancer therapeutics against solid tumors with historically poor prognosis.
Reference
1. Takebe N, L M, Harris P, Jeong W, Bando H, Kahn M, et al. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nature reviews Clinical oncology. 2015;12(8).
2. Sheeba CJ, Marslin G, Revina AM, Franklin G. Signaling pathways influencing tumor microenvironment and their exploitation for targeted drug delivery. Nanotechnology Reviews. 2014;3(2):123-51.
3. Meurette O. Shaping of the Tumor Microenvironment by Notch Signaling. Adv Exp Med Biol. 2020;1223:1-16.
4. Ruan Y, Ogana H, Gang E, Kim HN, Kim YM. Wnt Signaling in the Tumor Microenvironment. Adv Exp Med Biol. 2021;1270:107-21.
5. Goulet CR, Pouliot F. TGFbeta Signaling in the Tumor Microenvironment. Adv Exp Med Biol. 2021;1270:89-105.
6. Liu S, Ren J, Ten Dijke P. Targeting TGFbeta signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):8.
7. Grauel AL, Nguyen B, Ruddy D, Laszewski T, Schwartz S, Chang J, et al. TGFbeta-blockade uncovers stromal plasticity in tumors by revealing the existence of a subset of interferon-licensed fibroblasts. Nat Commun. 2020;11(1):6315.
8. NITHIKOON A, PITHI C. Integrin as a Molecular Target for Anti-cancer Approaches in Lung Cancer. Anticancer Research. 2019;39(2):541-8.
9. Seshacharyulu P, Ponnusamy MP, Haridas D, Jain M, Ganti AK, Batra SK. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16(1):15-31.
10. Katoh M. Fibroblast growth factor receptors as treatment targets in clinical oncology. Nat Rev Clin Oncol. 2019;16(2):105-22.
11. Katoh M, Nakagama H. FGF receptors: cancer biology and therapeutics. Med Res Rev. 2014;34(2):280-300.
12. Moosavi F, Giovannetti E, Peters GJ, Firuzi O. Combination of HGF/MET-targeting agents and other therapeutic strategies in cancer. Crit Rev Oncol Hematol. 2021;160:103234.
This web search service is supported by Google Inc.
