
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order. Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
 Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!  Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!
ACROBiosystems employs an application-oriented development strategy, with a particular focus on product design, quality control and solution-based support. We consider the end-user application of the product to carry out targeted product design. To ensure that the recombinant protein is as close to its natural state (multimeric complexes) as possible, we refer to structural characteristics of the protein during development. ACRO has successfully developed high-purity and high-quality CTLA4 dimer, IL-2Rβγ heterodimer, IL-2Rαβγ heterotrimer (patent application), TNFSFs trimer, etc.
An example illustrating ACRO’s structure guided design is in the development of Interleukin-2 receptor (IL-2R) complex construction.
In-vivo Interleukin-2 (IL-2) exerts its pleiotropic activities in promoting the proliferation, differentiation, and survival of mature T and B cells as well as the cytolytic activity of natural killer (NK) cells through binding to its heterotrimeric receptor complexes. Crystal structure of IL-2/IL-2R complex reveals that the alpha chain does not make any contact with either β or γc chains as shown. Key components (or combination) of these IL-2 receptor complexes are listed below showing a wide range of binding affinity.
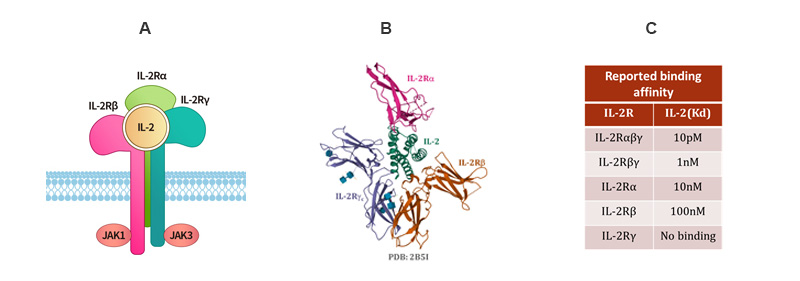
Figure 1. Structure and mechanism of IL-2/IL-2R interaction. A) Schematic of IL2/IL2R activation. B) Crystal structure of IL2/IL-2R complex. C) Dissociation constant of IL2 binding to IL-2R and components.(1, 2)
To design a stable IL-2R heterotrimer in-vitro, the γc and β subunits were induced to form heterodimer by heterodimerized Fc design while the C-terminus of a chain was linked covalently to N terminus of γc through a flexible linker of specific length designed to maintain the spatial complex conformation and IL-2 binding property.
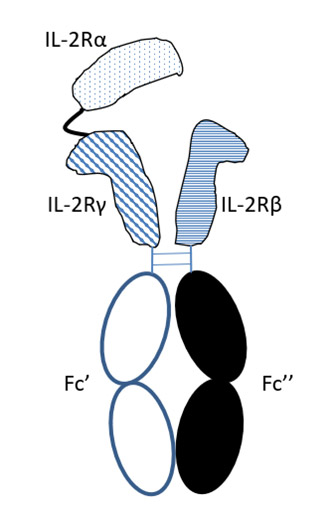
Figure 2. Design of IL-2Rαβγ heterotrimer
The complex was purified to greater than 90% purity.
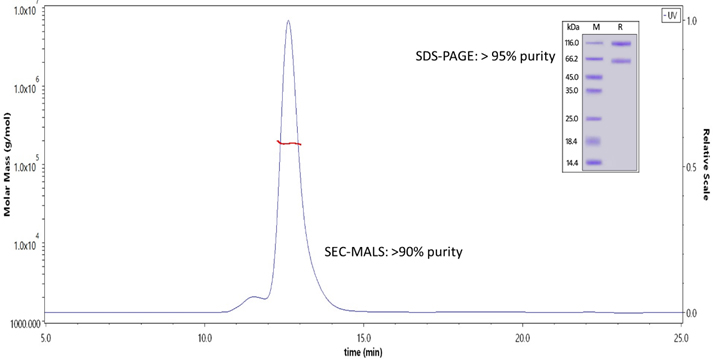
Figure 3. The purity of Human IL-2RB&IL-2RA&IL-2RG, Fc Tag&Fc Tag(Cat. No. ILG-H5257 ) is more than 90% and the molecular weight of this protein is around 175-190 kDa verified by SEC-MALS.
Binding study using ACRO’s IL-2Raβγc heterotrimer and IL2Rβγc construct revealed IL-2 binding comparable to native complex in SPR studies (Fig. 4). As controls, IL-2 binding affinity to individual chains of IL-2R were determined to also be similar to reported values.
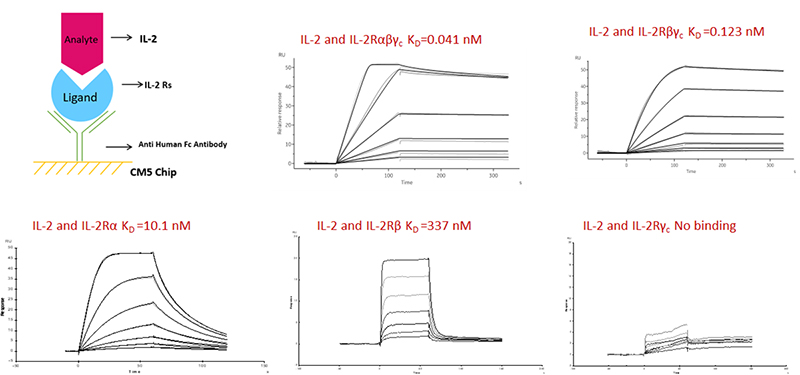
Figure 4. SPR determined binding affinities of IL-2 with ACRO’s IL2R constructs.
To ensure that the recombinant protein is as close to its natural state (multimeric complexes) as possible, we refer to structural characteristics of the protein during development. ACRO has successfully developed high-purity and high-quality Spike trimer , CTLA4 dimer , IL-2Rβγ heterodimer, IL-2Rαβγ heterotrimer (patent application), TNFSFs trimer , etc.
1. T. Waldmann, The shared and contrasting roles of IL2 and IL15 in the life and death of normal and neoplastic lymphocytes: implications for cancer therapy. Cancer immunology research 3, (2015).
2. X. Wang, M. Rickert, K. Garcia, Structure of the quaternary complex of interleukin-2 with its alpha, beta, and gammac receptors. Science 310, (2005).
This web search service is supported by Google Inc.








