Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order. Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
 Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!  Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!
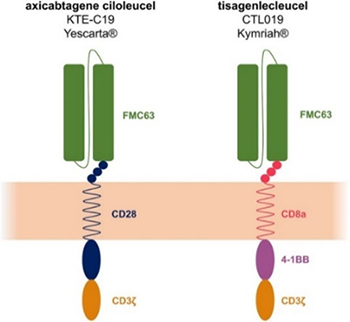
> Insights > Accurate evaluation of CD19 CAR expression in the quality control and clinical trials Chimeric antigen receptor T cell therapy (CAR-T cell therapy) is a novel immunotherapy with promising results in the treatment of hematological malignancies, and CD19 is currently the most widely used target in CAR-T cell therapy. CD19 CAR T cell therapy has been validated to be effective and safe for the treatment of B-ALL, CLL, and B cell lymphoma. So far, there are four CD19 targeted CAR-T cell therapy drugs have already been approved by FDA: Kymriah, Yescarta, Tecartus and Breyanzi. It is essential to evaluate CAR expression in the production of CAR-T cells and to monitor the persistence after administration. Here we show two powerful tools for detection of CD19 CAR expression at the stage of quality control and clinical trials.
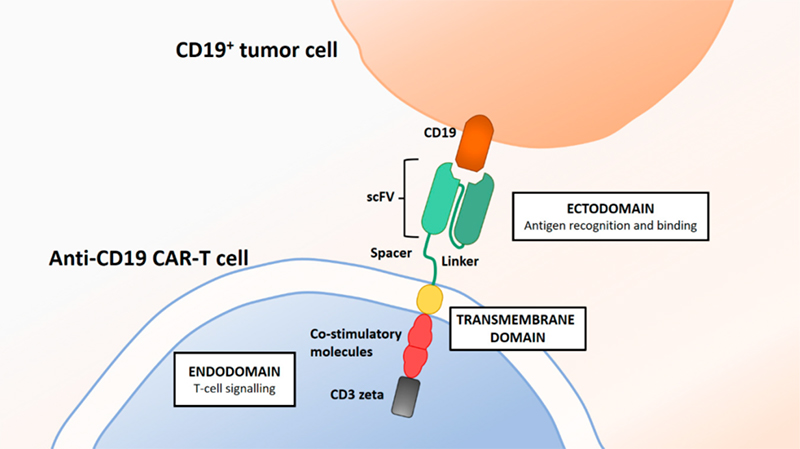 Britten, Oliver et al. 2019.
Britten, Oliver et al. 2019.
CD19 protein can specifically bind to the antigen-binding domains of its CARs and evaluate CAR functionality which makes CD19 an ideal reagent for CD19 CAR detection in the quality control. In addition, FMC63 is an IgG2a mouse monoclonal antibody targeting CD19. And the most of the reported CART19 trials test the anti-CD19 scFv derived from FMC63, including the four FDA-approved CD19 CARs. Anti-FMC63 antibody which can specifically recognize the scFv of anti-CD19 CARs derived from FMC63 has comparatively higher sensitivity and specificity. Therefore, anti-FMC63 antibody can be a preferrable option for the clinical samples with lower positive rate. However, the development of high-affinity CD19 protein and anti FMC63 antibody always face a big challenge and there are only a few products commercially available so far.
ACROBiosystems has developed a comprehensive series of CD19 protein products, including unconjugated CD19, biotinylated CD19 and fluorescent-labeled CD19. These proteins can effectively detect the expression of anti-CD19 CAR on the surface of the transduced T cells and have been widely used for the quality control release testing and pharmacokinetic (PK) study of anti-CD19 CAR-T cells. Meanwhile, ACRO has also developed the high-qualitied monoclonal anti-FMC63 scFv antibodies, the performance of which has been well validated in-house by flow cytometry, demonstrating suitable for detection of FMC63 derived anti-CD19 CARs in clinical trials.
PE Labeled CD19 can specifically detect CD19 CAR expression by FACS
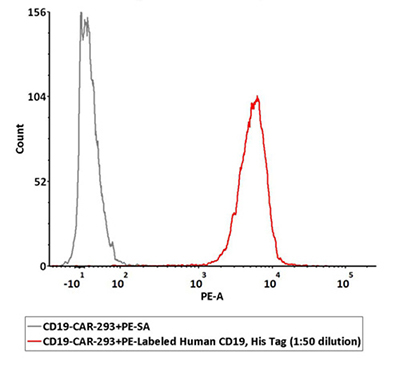
1e6 of the anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H3). PE Streptavidin was used as negative control.
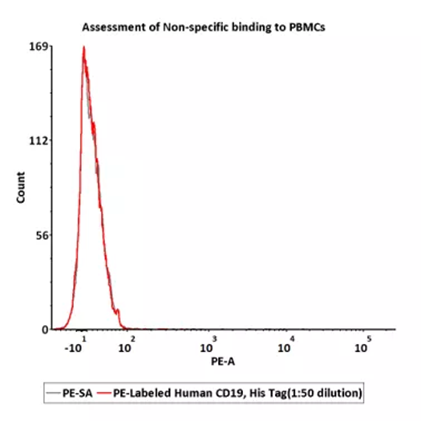
5e5 of PBMCs were stained with 100ul of 1:50 dilution of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H3), PE signal was used to evaluate the binding activity.
Higher binding affinity than competitive products verified by ELISA and FACS
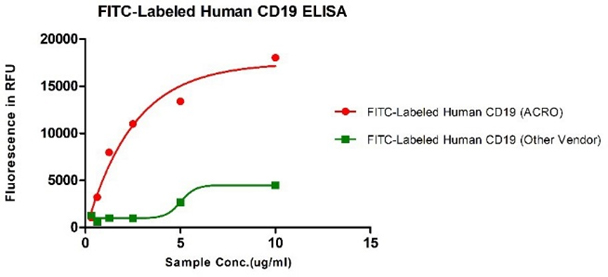
Human CD19, His Tag from two different vendors were evaluated in the ELISA analysis against FMC63 Mab. The result showed that ACRO's FITC-Labeled Human CD19, His Tag has a much higher binding activity than that of the other vendor.
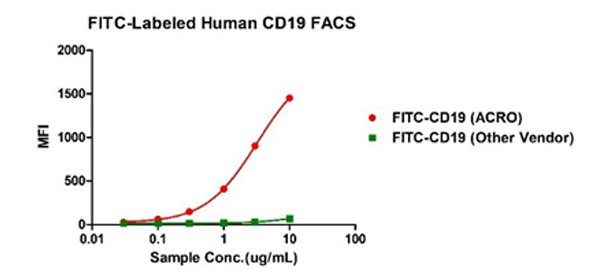
Binding activity of FITC-Labeled Human CD19, His Tag from two different vendors were evaluated in the flow cytometry analysis against anti-CD19-CAR-293 cells. The result showed that ACRO's FITC-Labeled Human CD19, His Tag has a much higher binding activity than that of the other vendor.
No background staining when detecting FMC63 derived Anti-CD19 CARs in clinical trials
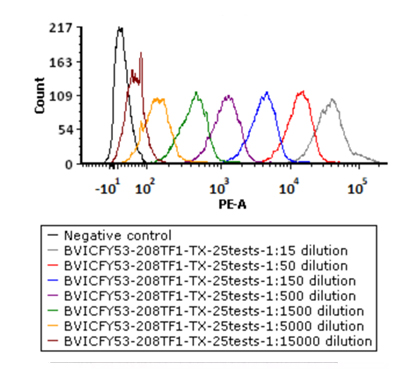
5e5 of Anti-CD19 CAR-293 cells were stained with a series of concentrations of PE-Labeled Monoclonal Anti FMC63 scFv Antibody, Mouse IgG1(Cat. No. FM3-HPY53) and negative control respectively. PE signal was used to evaluate the binding activity.
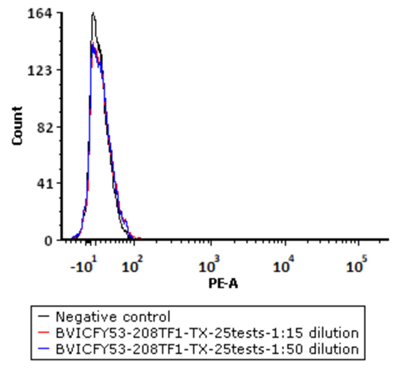
5e5 of Human PBMC cells were stained with a series of concentrations of PE-Labeled Monoclonal Anti FMC63 scFv Antibody, Mouse IgG1(Cat. No. FM3-HPY53) and negative control respectively. PE signal was used to evaluate the binding activity.
Stable at 37℃ for 72h, 4 ℃ for 14 days and -70℃ for 6 years with high bioactivity
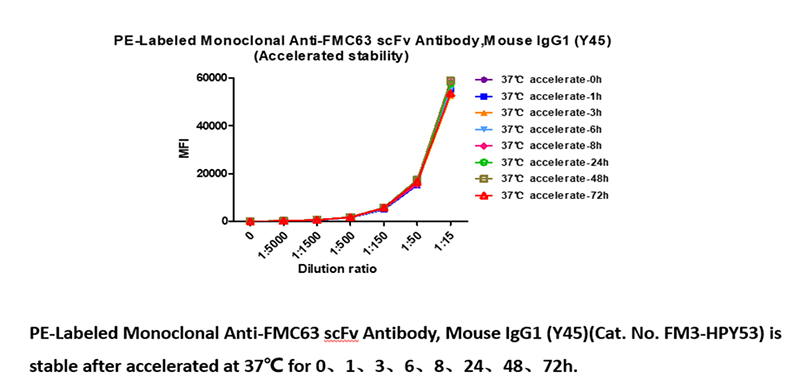
PE-Labeled Monoclonal Anti-FMC63 scFv Antibody, Mouse IgG1 (Y45)(Cat. No. FM3-HPY53) is stable in undiluted samples at 37℃ for 72 hours, equivalent to store at 4 ℃ for 14 days without performance reduction and equivalent to store at -70℃ for 83 months without performance reduction.
Binding activity of different lots of anti-FMC63 antibody were verified by FACS
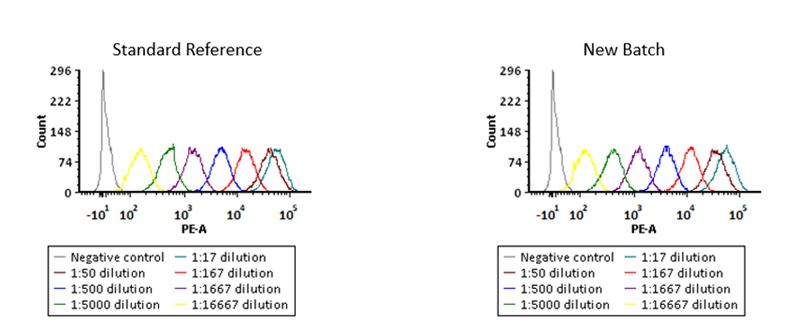
Binding activity of two different lots of PE-Labeled Monoclonal Anti FMC63 scFv Antibody, Mouse IgG1(Cat. No. FM3-HPY53) against Anti-CD19 CAR-293 cells was evaluated by flow cytometry. The result shows very high batch-to-batch consistency.
CAR-T cells product is a kind of a living drug, and it requires whole-process quality control, such as inspection of the materials used in production, process control, and a release test of the finished products. Evaluating CAR expressing is an essential step in the quality control of CAR-T cells. ACRO’s CD19 proteins and anti FMC63 antibody products with high specificity and sensitivity are ideal tools for the quality control release testing and pharmacokinetic study.
[1] Abramson JS. Anti-CD19 CAR T-Cell Therapy for B-Cell Non-Hodgkin Lymphoma. Transfus Med Rev. 2020;34(1):29-33.
[2] Britten O, Ragusa D, Tosi S, Kamel YM. MLL-Rearranged Acute Leukemia with t (4;11) (q21;q23)-Current Treatment Options. Is There a Role for CAR-T Cell Therapy?. Cells. 2019;8(11):1341.
[3] Cerrano M, Ruella M, Perales MA, et al. The Advent of CAR T-Cell Therapy for Lymphoproliferative Neoplasms: Integrating Research Into Clinical Practice. Front Immunol. 2020;11:888.
This web search service is supported by Google Inc.