
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order. Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
 Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!  Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!

This protein carries a polyhistidine tag at the C-terminus.
The protein has a calculated MW of 44.6 kDa.
PE
Excitation Wavelength: 488 nm / 561 nm
Emission Wavelength: 575 nm
Lyophilized from 0.22 μm filtered solution in PBS, 0.2% BSA, pH7.4. Normally trehalose is added as protectant before lyophilization.
Contact us for customized product form or formulation.
Please see Certificate of Analysis for specific instructions.
For best performance, we strongly recommend you to follow the reconstitution protocol provided in the CoA.
For long term storage, the product should be stored at lyophilized state at -20°C or lower.
Please protect from light and avoid repeated freeze-thaw cycles.
This product is stable after storage at:


5e5 of anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H5) and negative control protein respectively (Fig. C and B), and non-transfected 293 cells were used as a control (Fig. A). PE signal was used to evaluate the binding activity (QC tested).

5e5 of PBMCs were stained with PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H5) and anti-CD3 antibody, washed and then analyzed with FACS. FITC signal was used to evaluate the expression of CD3+ T cells in PBMCs, and PE signal was used to evaluate the non-specific binding activity to PBMCs (QC tested).
Please contact us via TechSupport@acrobiosystems.com if you have any question on this product.

Explore our catalog of therapeutic antibody solutions to find the right products for you! We are dedicated to delivering solutions designed to help you drive innovation and push the boundaries of what therapeutic antibodies can be.

Organoid Toolbox is a collection of organoid solutions including ready-to-use organoids, organoid differentiation kits, and a variety of services to accelerate the progress of your drug development project.
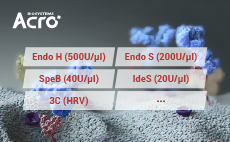
To enable antibody characterization methodsACROBiosystems has developed a series of enzymes.such as ldeS, SpeB, EndoH, and Endo S proteases, toassist with the characterization of antibodies and theirrelated post-translational modifications (PTMs)

ACROBiosystems developed a series of GMP grade cytokines under the GMP grade quality management system. Those products are all suitable for T/NK cell generation, activation, and proliferation in cell therapy research.

50+ targets designed for CAR detection, including PE/FITC/biotin labeled proteins. The key reagents for CD19 and BCMA were FDA DMF filed which can support your IND, NDA and BLA process.

GMP grade cytokines, reagents for cell activation, gene edition, DNA/RNA removal, etc. Particularly focus on product design, quality control and solution-based support to link each phase of your cell and gene therapy journey.

Full length multi-pass TPs with stabilized structure and high bioactivity for immunization, antibody screening, cell based assay and CAR detection, including hot CD20, Claudin 18.2, CD133, GPRC5D,CCR8, CCR5, etc.

A series of immune checkpoints including classic co-inhibitory and co-stimulatory receptors. The comprehensive catalog contains 100+ targets with various species and tags, and the high-quality proteins are in good batch-to-batch consistency.

To meet the needs of ADCs development, ACROBiosystems can provide: A variety of high-quality target proteins; MMPs/Cathepsin/uPA for cleavable linker; Anti-payload antibodies & anti-idiotypic antibodies for immunogenicity and PK analysis; SPR/BLI analytical and ADA development service.

Comprehensive collection of Fc receptor proteins, including their common variants, which can help expedite your antibody development.

Comprehensive cytokine targets including interleukins, growth factors, chemokines, TNFs, etc. are expressed by HEK293 to ensure their natural structure. Their high purity is verified by SDS-PAGE/HPLC/SEC-MALS and high bioactivity is verified by ELISA/SPR/BLI.

Aneuro provides innovative solutions for neuroscience research. Recombinant proteins, neural factors, pre-formed fibrils, electrophysiological electrodes, as well as Organoid Toolbox all in Aneuro aiming to advance neuroscience research, develop therapeutic interventions, and improve diagnostic methods for neurological diseases.
| English Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Axicabtagene ciloleucel | FKC-876; KTE-C19; KTE C19 | Approved | Cabaret Biotech, Gilead Sciences Inc | Yescarta, 奕凯达 | United States | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, B-Cell | Kite Pharma Eu Bv | 2017-10-18 | Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Primary mediastinal B cell lymphoma; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin | Details |
| Tafasitamab | XENP-5574; MOR-00208; XmAb-5574; MOR-208 | Approved | Xencor Inc | MONJUVI, Minjuvi | United States | Lymphoma, Large B-Cell, Diffuse | Morphosys Us Inc | 2020-07-31 | Lymphoma, Large B-Cell, Diffuse; Graft vs Host Disease; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Hemic and Lymphatic Diseases; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Blinatumomab | MEDI-538; MT-103; BiTE-MT-103; bscCD19xCD3; AMG-103 | Approved | Amgen Inc | Blincyto, 倍利妥 | United States | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Amgen Inc | 2014-12-03 | Neoplasm, Residual; Leukemia, Myelogenous, Chronic; Leukemia; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Multiple Myeloma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor T-Cell Lymphoblastic Leukemia-Lymphoma; Philadelphia Chromosome; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Lymphoma; Burkitt Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Tisagenlecleucel | LG-740; CART-019; CTL-019; CART-19 | Approved | Novartis Pharma Ag, University Of Pennsylvania | Kymriah | United States | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Novartis Pharmaceuticals Corp | 2017-08-30 | Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Large B-Cell, Diffuse; Multiple Myeloma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Plasmablastic Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Lymphoma; Central Nervous System Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Leukemia, Prolymphocytic | Details |
| Inaticabtagene Autoleucel | CNCT19; CNCT-19; HY001 | Approved | Juventas Cell Therapy Ltd | 源瑞达 | Mainland China | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Juventas Cell Therapy Ltd | 2023-11-08 | Hematologic Neoplasms; Lymphoma, B-Cell; Purpura, Thrombocytopenic, Idiopathic; Lymphoma, Large B-Cell, Diffuse; Lupus Nephritis; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lupus Erythematosus, Systemic; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| Inebilizumab | VIB-0551; 16C4-aFuc; MEDI-551; MT-0551 | Approved | Duke University, Horizon Therapeutics PLC | Uplinza, Uplizna, ユプリズナ | United States | Neuromyelitis Optica | Viela Bio Inc | 2020-06-11 | Hematologic Neoplasms; Myasthenia Gravis; Lymphoma, B-Cell; Rejection of renal transplantation; Immunoglobulin G4-Related Disease; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Scleroderma, Systemic; Multiple Sclerosis; Neuromyelitis Optica; Leukemia, B-Cell; Leukemia, Lymphocytic, Chronic, B-Cell; Encephalitis | Details |
| Brexucabtagene autoleucel | KTE-X19; FKC-889 | Approved | Kite Pharma Inc, Gilead Sciences Inc | Tecartus | United States | Lymphoma, Mantle-Cell | Kite Pharma Inc | 2020-07-24 | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Relmacabtagene autoleucel | JWCAR-029 | Approved | Suzhou Yaomingjunuo Biotechnology Co Ltd | 倍诺达, Carteyva | Mainland China | Lymphoma, Large B-Cell, Diffuse | Jw Therapeutics (Shanghai) Co Ltd | 2021-09-01 | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lupus Erythematosus, Systemic; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Lisocabtagene maraleucel | Liso-Cel; JCAR-017 | Approved | Memorial Sloan Kettering Cancer Center, Fred Hutchinson Cancer Research Center, Juno Therapeutics Inc, Seattle Children'S Research Institute | Breyanzi | United States | Lymphoma, Large B-Cell, Diffuse | Bristol-Myers Squibb Company | 2021-02-05 | Lymphoma, B-Cell; Immunoproliferative Disorders; Lymphatic Diseases; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Mediastinal Neoplasms; Immune System Diseases; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoproliferative Disorders; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Loncastuximab tesirine | ADCT-402; MT-2111 | Approved | Adc Therapeutics Sa | ZYNLONTA, Lonca | United States | Lymphoma, Large B-Cell, Diffuse | Adc Therapeutics Sa | 2021-04-23 | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lymphoproliferative Disorders; Lymphoma, Non-Hodgkin; Waldenstrom Macroglobulinemia; Burkitt Lymphoma; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| English Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| 4G7SDIE | Clinical | Synimmune Gmbh, University Of Tubingen | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Fully human anti CD19 CAR-T Cell Therapy (University Hospitals Cleveland Medical Center) | Phase 1 Clinical | University Hospitals Cleveland Medical Center | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Anti-CD19/22-CAR vector-transduced T cell therapy (Chinese PLA General Hospital) | Phase 2 Clinical | People'S Liberation Army General Hospital Military Service | Leukemia; Lymphoma | Details | |
| Anti-CD22/CD19 monoclonal antibody-toxin conjugate | Phase 1 Clinical | The University Of Texas Southwestern Medical Center | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| ThisCART-19 | ThisCART-19; ThisCART19A; ThisCART19C; ThisCART19B | Phase 2 Clinical | Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, AIDS-Related; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Lymphoma | Details | |
| Anti-CD20 CAR T-cell therapy (Southwest Hospital Chongqing) | Phase 2 Clinical | Southwest Hospital Chongqing | Lymphoma, Large B-Cell, Diffuse | Details | |
| aCD19z | aCD19z | Phase 1 Clinical | Christie Hospital Nhs Foundation Trust | Lymphoma, Non-Hodgkin | Details |
| PTG-01 | PTG-01 | Phase 1 Clinical | Protheragen | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| TC-110 | TC-110 | Phase 2 Clinical | Tcr2 Therapeutics Inc | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| pCAR-19B | Phase 2 Clinical | Chongqing Precision Biotechnology Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| H-31970-SAGAN | H-31970-SAGAN | Phase 1 Clinical | Baylor College Of Medicine | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD19 CAR T-cell therapy (Sheba Medical Center) | Phase 3 Clinical | Chaim Sheba Medical Center At Tel Hashomer | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute | Details | |
| Anti-CD19 CAR-T cell therapy (Shenzhen Geno-Immune Medical Institute) | 4SCAR-19 | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell | Details |
| CAR-20-19-22 | CAR-20-19-22 | Phase 1 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Zamtocabtagene autoleucel | MB-CART2019.1 | Phase 2 Clinical | Miltenyi Biotec | Lymphoma, Large B-Cell, Diffuse | Details |
| CD19 CAR-T (HuaDao CAR-Tcell) | Phase 2 Clinical | Huadao (Shanghai) Biopharma Co Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| OnCARlytics(Imugene) | CF-33-CD-19 | Phase 1 Clinical | City Of Hope National Medical Center, Imugene Ltd | Solid tumours | Details |
| GC-022 | GC-022; GC-022F | Phase 2 Clinical | Hematologic Neoplasms; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| CD19-CART (Unicar-Therapy Bio-medicine Technology) | Phase 1 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CD19/CD20 bispecific chimeric antigen receptor (CAR)-T cell therapy (Jonsson Comprehensive Cancer Center) | Phase 1 Clinical | Uclas Jonsson Comprehensive Cancer Center | Candidiasis, Vulvovaginal; Lymphoma, B-Cell; Leukemia, Lymphoid; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| CD19/CD20 CAR-T Cell Therapy (PersonGen) | Phase 1 Clinical | Persongen Biotherapeutics | Details | ||
| CD19- CD34t metabolically programmed CAR T-cell therapy (Medical University of South Carolina) | Phase 2 Clinical | Medical University Of South Carolina | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| CD19-CART (Express 4-1BB, Unicar-Therapy Bio-medicine Technology) | Phase 2 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Candidiasis, Vulvovaginal; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Anti-CD19 CAR-T therapy (Wuhan Sian Medical Technology) | Phase 3 Clinical | Wuhan Si'an Medical Technology Co Ltd, Xiangyang Central Hospital, Jingzhou Central Hospital, Wuhan Union Hospital, The First People'S Hospital Of Yuhang District | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Burkitt Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| CD19/CD20 bispecific CAR-T cells (Shanghai Cellular) | Phase 2 Clinical | Shanghai Cellular Biopharmaceutical Group Ltd | Lymphoma, B-Cell | Details | |
| Anti-BCMA/CD19 CAR-T Therapy(University College London) | Phase 1 Clinical | University College London | Multiple Myeloma | Details | |
| BCN-CP01 | BCN-CP01; BCN CP01; BCN-CP-01; GLPG5201; GLPG-5201 | Phase 2 Clinical | CellPoint BV | Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD19 Autologous CAR-T Cell therapy (920th Hospital) | OlyCAR-019 | Phase 2 Clinical | The 920th Hospital Of Joint Logistics Support Force Of PLA | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| CD19-CD22 CAR-T cells (Shanghai Ultra-T Immune Therapeutics) | Phase 2 Clinical | Shanghai Ultra-T Immune Therapeutics Co LTD | Leukemia, B-Cell | Details | |
| TBI-2001 | TBI-2001 | Phase 1 Clinical | University Health Network, Takara Bio Inc | Lymphoma, B-Cell; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Metabolically Armed CD19 CAR-T cells(Leman Biotech) | Meta10-19 | Phase 1 Clinical | Leman Biotech Co Ltd, Anhui Provincial Hospital | Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| 4SCAR19U T Cells Therapy (Shenzhen Geno-Immune Medical Institute) | 4SCAR19U cells | Phase 1 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| Autologous CD19/CD22 chimeric antigen receptor T-cell therapy (MD Anderson Cancer Center) | Phase 2 Clinical | The University Of Texas MD Anderson Cancer Center | Candidiasis, Vulvovaginal; Neoplasm, Residual; Lymphoma, B-Cell; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Anti-CD19 CAR T-cell therapy (Fujian Medical University) | Phase 3 Clinical | Fujian Medical University | Lymphoma, B-Cell | Details | |
| MB-CART19.1 (Miltenyi Biotec) | Phase 2 Clinical | Miltenyi Biotec | Lymphoma, B-Cell; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| XYF19 CAR-T Cell Therapy | XYF-19 | Phase 1 Clinical | Guangzhou Yinming Biomedical Technology Co Ltd, Xi'An Yufan Biotechnology Co Ltd | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| huCART19-IL18 | Phase 1 Clinical | University Of Pennsylvania, Kite Pharma | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| CD19 chimeric antigen receptor T cell therapy (Takara Bio) | TBI-1501 | Phase 2 Clinical | Memorial Sloan Kettering Cancer Center, Takara Bio Inc | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| CD3/CD19 neg allogeneic BMT (National Institute of Allergy and Infectious Diseases/University of Pittsburgh) | Phase 2 Clinical | National Institute Of Allergy And Infectious Diseases (Niaid), University Of Pittsburgh | Primary Immunodeficiency Diseases; Female Urogenital Diseases; Inflammation | Details | |
| BCMA-CD19 cCAR T cell therapy (iCell Gene Therapeutics) | Phase 1 Clinical | Icell Gene Therapeutics (Int'L) Ltd | Multiple Myeloma; Lupus Erythematosus, Systemic; Waldenstrom Macroglobulinemia | Details | |
| Virus-specific-CD19.CAR | Phase 1 Clinical | Baylor College Of Medicine | Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Anti-CD19 CAR T-cell therapy (University College London) | 4G7-CARD | Phase 1 Clinical | University College London | Central Nervous System Lymphoma | Details |
| Anti-CD19 CAR T-cell therapy (Southwest Hospital Chongqing) | Phase 2 Clinical | Southwest Hospital Chongqing | Lymphoma, Large B-Cell, Diffuse | Details | |
| CD19.CAR-VST | CD19.CAR-VST | Phase 1 Clinical | Baylor College Of Medicine | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| C-CAR011 | Phase 2 Clinical | Pla General Hospital | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details | |
| CD19-CAR(The University Of Texas MD Anderson Cancer Center) | Phase 1 Clinical | The University Of Texas MD Anderson Cancer Center | Leukemia; Lymphoma | Details | |
| CD19 CAR-T cell therapy (Chongqing Precision Biotech) | MC-1-50 | Phase 2 Clinical | Chongqing Precision Biotechnology Co Ltd | Lymphoma, B-Cell; Leukemia; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Dermatomyositis; Lymphoma, Large B-Cell, Diffuse; Sjogren's Syndrome; Scleroderma, Systemic; Lupus Erythematosus, Systemic; Leukemia, B-Cell; Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Rapcabtagene autoleucel | YTB-323 | Phase 2 Clinical | Novartis Pharma Ag | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lupus Nephritis; Lupus Erythematosus, Systemic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Evoncabtagene pazurgedleucel | CTX-110 | Phase 2 Clinical | Crispr Therapeutics Ag | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| Cord Blood-derived CAR NK Cells Targeting CD19/CD70(Tongji University) | Tongji University | Details | |||
| Anti-CD19-CAR T cell therapy (Kecellitics Biotech) | ORGCAR19; ORGCAR-19 | Phase 2 Clinical | Kecellitics Biotech Company Ltd | Lymphoma, B-Cell; Leukemia; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma | Details |
| Anti-CD19 CAR T-cell therapy (Leidos Biomedical Research) | Clinical | Leidos | Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| CD19-targeted chimeric antigen receptor T-cell(Pell Bio-Med Technology) | PL-001 | Phase 2 Clinical | Pell Bio-Med Technology Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Primary mediastinal B cell lymphoma; Lymphoma, Follicular; Lymphoma, Non-Hodgkin | Details |
| PCAR-019 (Anke Biotechnology) | Phase 1 Clinical | Anhui Anke Biotechnology (Group) Co Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Prolymphocytic, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| A-319 | A-319 | Phase 1 Clinical | Evive Biotech Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details |
| CD19/79b Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | bi-4SCAR CD19/79b | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| CB-010 | Phase 1 Clinical | Caribou Biosciences Inc | Lymphoma, B-Cell; Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| CD19/CD22 CAR-T cell therapy (Federal Research Institute of Pediatric Hematology, Oncology and Immunology) | Phase 2 Clinical | Federal Research Institute of Pediatric Hematology Oncology and Immunology | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Burkitt Lymphoma | Details | |
| Dual anti-CD19/CD22 CAR‐T cell therapy (B-cell malignancies, Stanford University) | Phase 1 Clinical | Stanford University, Orca Biosystems Inc | Leukemia, Myelogenous, Chronic; Neoplasm, Residual; Leukemia, Lymphoid; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Prizloncabtagene autoleucel (AbelZeta Pharma) | C-CAR039; C-CAR-039; C CAR 039; EXP-039 | Phase 2 Clinical | Cellular Biomedicine Group Ltd | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| JCAR-014 | JCAR-014; JCAR021 | Phase 2 Clinical | Fred Hutchinson Cancer Research Center, Memorial Sloan Kettering Cancer Center, Seattle Children'S Research Institute, Juno Therapeutics Inc | Lymphoma, B-Cell; Leukemia; Leukemia, Lymphoid; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Candidiasis; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| CABA-201 | CABA-201 | Phase 2 Clinical | Cabaletta Bio Inc | Myasthenia Gravis; Myositis; Dermatomyositis; Autoimmune Diseases; Scleroderma, Systemic; Lupus Nephritis; Lupus Erythematosus, Systemic; Muscular Diseases | Details |
| Varnimcabtagene autoleucel | ARI-0001; IMN-003A; IMN-003-A | Phase 2 Clinical | Hospital Clínic De Barcelona, Institut D'Investigacions Biomèdiques August Pi I Sunyer | Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| CD79b-19 CAR T Cells Therapy (Massachusetts General Hospital) | Massachusetts General Hospital | Details | |||
| Anti-human CD19-CD22 T cell therapy | Phase 1 Clinical | Hrain Biotechnology Co Ltd | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Anti-CD19 CAR T cell therapy (Wuhan Bio-Raid) | Phase 2 Clinical | Hematologic Diseases; Lymphoma, B-Cell | Details | ||
| Anti-CD19 CAR-T cell therapy (Uppsala University) | Phase 2 Clinical | Uppsala University | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| CD19-CD123 cCAR T-cells Therapy(iCell Gene Therapeutics) | ICG140 | iCell Gene Therapeutics LLC | Details | ||
| SC-291 | SC-291 | Phase 1 Clinical | Sana Biotechnology Inc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| HuCART19 | Phase 2 Clinical | National Cancer Institute, Kite Pharma | Lymphoma, B-Cell; Rejection of renal transplantation; Renal Insufficiency; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Kidney Failure, Chronic | Details | |
| CD19 CAR-T cell therapy (The First Affiliated Hospital of Soochow University) | Phase 2 Clinical | The First Affiliated Hospital Of Soochow University, Suzhou University | Leukemia; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CD19-targeting humanized selective CAR-T cell therapy (Xuanwu Hospital, Beijing) | Phase 2 Clinical | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | ||
| Anti-CD19-CAR T cells (Beijing Doing Biomedical) | Phase 1 Clinical | Beijing Doing Biomedical Co Ltd | Leukemia | Details | |
| Anti-CD19 CAR-T cell therapy (Japan Tissue Engineering) | Phase 1 Clinical | Nagoya University, Shinshu University | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CAR-CD19 T cell therapy | Phase 2 Clinical | Carsgen Biomedicine (Shanghai) Co Ltd | Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Lymphoma | Details | |
| AMG-562 | AMG-562 | Phase 1 Clinical | Amgen Inc | Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell | Details |
| Anti-CD19 CAR-T cell therapy (Pinze Lifetechnologies) | PZ-01 | Clinical | Pinze Lifetechnology Co Ltd | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| MCM-998 | MCM-998; LXG-250 | Phase 1 Clinical | Novartis Pharma Ag | Multiple Myeloma | Details |
| CLIC-1901 (Ottawa Hospital Research Institute) | CLIC-1901 | Phase 2 Clinical | Ottawa Hospital Research Institute | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Obexelimab | XmAb-5871; AMG-729 | Phase 3 Clinical | Xencor Inc | Immunoglobulin G4-Related Disease; Lupus Erythematosus, Systemic | Details |
| dPD-1 hCD19 CART cell therapy (Innovative Cellular Therapeutics) | ICTCAR-014 | Phase 2 Clinical | Innovative Cellular Therapeutics Co Ltd | Lymphoma, Non-Hodgkin | Details |
| CTA-101 | CTA-101 | Phase 1 Clinical | Nanjing Bioheng Biotech Co Ltd, Nanjing Medical University, Xuzhou Medical University (Xzmu) | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| 1928-zT-2 (Guangzhou Institutes of Biomedicine and Health) | Phase 1 Clinical | Guangzhou Institute Of Biomedicine And Health, Chinese Academy Of Sciences | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| TanCART19/20 | Phase 1 Clinical | Pla General Hospital | Neuromyelitis Optica | Details | |
| Dual specificity CD19 and CD20 or CD22 CAR-T cell therapy(Chinese PLA General Hospital) | Phase 2 Clinical | Pla General Hospital | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| CD19/22 Bi-specific CAR-T Cell Therapy(Shenzhen Geno-Immune Medical Institute) | 4SCAR19/22 | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| Autologous humanized anti-CD19 and anti-CD20 dual specific CAR-T cell therapy (First Affiliated Hospital of Zhejiang University) | Phase 1 Clinical | First Affiliated Hospital Of Zhejiang University | Lymphoma, Large B-Cell, Diffuse | Details | |
| NCAR19-T | Phase 2 Clinical | Second Military Medical University Of Chinese People'S Liberation Army | Lymphoma, B-Cell | Details | |
| AZD-0486 | TNB-486; AZD-0486; AZD0486 | Phase 2 Clinical | Teneobio Inc, Astrazeneca Plc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin | Details |
| GC-007g | GC007g; GC-007g | Phase 2 Clinical | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details | |
| IM19CAR-T | IM19 | Phase 2 Clinical | Beijing Yimiao Medical Technology Co Ltd | Leukemia; Hematologic Neoplasms; Neoplasms; Lymphoma, Mantle-Cell; Leukemia, B-Cell; Lymphoproliferative Disorders; Lymphoma, Non-Hodgkin; Lymphoma | Details |
| UTAA-09 cells therapy | UTAA-09; UTAA09; UTAA09/17γδT; UTAA 09/17 | Phase 1 Clinical | Persongen Biotherapeutics (Suzhou) Co Ltd, Anhui Provincial Hospital | Lymphoma, B-Cell | Details |
| CLN-978 | CLN-978 | Phase 1 Clinical | Adimab LLC | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| GC-007F (Gracell Biotechnology) | GC-007F | Phase 1 Clinical | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CD19 directed CAR T cell therapy (University of Colorado) | Phase 1 Clinical | University Of Colorado | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Anti-CD19/CD70 NK cell therapy (Zhejiang University) | Phase 1 Clinical | Zhejiang University | Lymphoma, Non-Hodgkin | Details | |
| Anti-CD19 CAR NK Cell therapy(Enricnk) | KN5501; KN-5501 | Phase 1 Clinical | Changhai Hospital | Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic | Details |
| Anti-CD19 CAR T-cell therapy (Hebei Senlang Biotechnology) | SENL-B19 | Phase 2 Clinical | Leukemia; Lymphoma, B-Cell; Multiple Myeloma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma | Details | |
| QH-103 Cell Therapy | QH-103 | Phase 1 Clinical | Anhui Provincial Hospital | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti-CD19 CAR-Engineered NK Cells therapy (Simnova) | CAR-NK-CD19 | Phase 1 Clinical | Shanghai Simnova Biotechnology Co Ltd | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lupus Erythematosus, Systemic; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| α/β CD3+/CD19+ cell depleted stem cell therapy (Mitchell Cairo) | Phase 2 Clinical | New York Medical College | Leukemia; Anemia; Thalassemia; Hodgkin Disease; Anemia, Aplastic; Thrombocytopenia; Lymphoma, Non-Hodgkin; Kostmann Syndrome; Anemia, Sickle Cell | Details | |
| CD19 Universal CAR-γδ T Cells Therapy(Guangzhou Bio-Gene Technology) | Phase 2 Clinical | Guangzhou Bio-Gene Technology Co Ltd, Wuhan Union Hospital | Lupus Erythematosus, Systemic | Details | |
| Anti-CD19 CAR T-cell therapy (Shenzhen Institute for Innovation and Translational Medicine) | yinnuokati-19 | Phase 2 Clinical | Dongguan People'S Hospital, Shenzhen Institute For Innovation & Translational Medicine | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD19 CAR T-cell therapy (Nanjing Medical University) | XLCART-001 | Phase 2 Clinical | Nanjing Medical University | Lymphoma, B-Cell | Details |
| Anti-CD19 CAR-T cells therapy (Tianjin Mycure Medical Technology) | Phase 1 Clinical | Tianjin Mycure Medical Technology Co Ltd | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| 3rd-gen-CD19-CAR | 3rd-gen-CD19-CAR | Phase 2 Clinical | Bluebird Bio Inc | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| 4-1BBz CD19-Her2tG (Seattle Children's Hospital) | Phase 1 Clinical | Seattle Children'S Hospital | Leukemia; Neoplasms; Lymphoma | Details | |
| CD19-PD1-CAR T cell therapy (Chinese PLA General Hospital) | Phase 2 Clinical | Pla General Hospital | Lymphoma, B-Cell | Details | |
| Allogenic Anti-CD19 gamma delta CAR-T cell therapy(PLA General Hospital) | Phase 2 Clinical | Pla General Hospital | Lymphoma, Non-Hodgkin | Details | |
| Anti-CD19 and anti-CD22 CAR T cell therapy (Seattle Children's Hospital) | Phase 1 Clinical | Seattle Children'S Hospital | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Anti-CD19/CD22 CAR-T cell therapy (Shenzhen BinDeBio) | Phase 1 Clinical | Dehe Biotech Co Ltd | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Autologous anti-CD19 CAR T cell therapy(Kyverna Therapeutics) | KYV-101 | Phase 2 Clinical | National Institutes Of Health, Kyverna Therapeutics Inc | Myasthenia Gravis; Anti-Neutrophil Cytoplasmic Antibody-Associated Vasculitis; Myositis; Multiple Sclerosis, Chronic Progressive; Multiple Sclerosis; Lupus Nephritis; Scleroderma, Diffuse | Details |
| ISIKOK-19 | ISIKOK-19 | Phase 2 Clinical | Acibadem University | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| RG-6333 | RO-7443904 | Phase 1 Clinical | F. Hoffmann-La Roche Ag | Lymphoma, Non-Hodgkin | Details |
| PBLTT52CAR19 | Phase 1 Clinical | Great Ormond Street Hospital For Children Nhs Foundation Trust | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CD19.CAR T Cells (Heidelberg University Hospital) | Phase 2 Clinical | Heidelberg University Hospital | Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Autologous CD19CAR-CD28-CD3zeta-EGFRt-expressing Tcm-enriched T cells (City of Hope Medical Center) | Phase 1 Clinical | City Of Hope National Medical Center | Lymphoma, Non-Hodgkin | Details | |
| ET019003-T Cells | ET-019003 | Phase 1 Clinical | Wuhan Union Hospital, Eureka Therapeutics Inc | Leukemia; Lymphoma | Details |
| CD19x22 CAR T Cell Therapy (University Of Colorado Denver) | Phase 1 Clinical | University Of Colorado, Denver, Usa | Lymphoma, Non-Hodgkin | Details | |
| K-193 | K-193 | Phase 1 Clinical | Beijing Lvzhu Biological Technology Co Ltd | Lymphoma, B-Cell | Details |
| IKS-03 | IKS-03 | Phase 1 Clinical | Iksuda Therapeutics | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Lymphoma, Non-Hodgkin | Details |
| BCMA/CD19 CAR-T cell therapy (The First Affiliated Hospital of Nanchang University) | Phase 1 Clinical | The First Affiliated Hospital Of Nanchang University | Multiple Myeloma | Details | |
| CD19 specific Chimeric Antigen Receptor T Cell therapy(Nationwide Children'S Hospital) | Phase 1 Clinical | Nationwide Children'S Hospital | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| CD19-CAR-T2 cell therapy (Nanfang Hospital of Southern Medical University) | Phase 2 Clinical | Nanfang Hospital Of Southern Medical University | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Biphenotypic, Acute | Details | |
| Anti-CD19 CAR-T Cells (Shaanxi Provincial People'S Hospital) | Clinical | Leukemia, B-Cell | Details | ||
| CD19-STAR-T cell therapy (China Immunotech Co Ltd) | Phase 1 Clinical | Hebei Yanda Hospital, China Immunotech Co Ltd | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| bispecific CD19/CD22 CAR T-cell therapy (University of Colorado) | Phase 1 Clinical | University Of Colorado | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| Autologous anti-CD19 chimeric antigen receptor T-cell therapy (Malaghan Institute of Medical Research) | WZTL002-1 | Phase 1 Clinical | The Malaghan Institute Of Medical Research | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Primary mediastinal B cell lymphoma; Lymphoma, Non-Hodgkin | Details |
| CD19/BCMA-targeted CAR-T Cell Therapy (Zhejiang University) | Phase 1 Clinical | Zhejiang University, Shanghai YaKe Biotechnology Co Ltd | Nephritis; Sjogren's Syndrome; Scleroderma, Systemic; Autoimmune Diseases; Multiple Myeloma; Lupus Nephritis; Lupus Erythematosus, Systemic; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| Anti-CD19 UCAR-NK cells(920th Hospital of Joint Logistics Support Force of People's Liberation Army of China) | Phase 2 Clinical | The 920th Hospital Of Joint Logistics Support Force Of PLA | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| Anti-CD19 chimeric antigen receptor T cell therapy(TriArm Therapeutics) | Phase 1 Clinical | TriArm Therapeutics (Shanghai) Co Ltd | Hematologic Neoplasms; Lymphoma, Non-Hodgkin | Details | |
| Anti-CD19 CAR-T cell therapy (Bellicum Pharmaceuticals) | BPX-401 | Phase 2 Clinical | Bellicum Pharmaceuticals Inc, Ospedale Pediatrico Bambino Gesu | Leukemia; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma | Details |
| CTA-30X | CTA-30X; CTA30X | Phase 1 Clinical | Nanjing Bioheng Biotech Co Ltd | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| CD19/CD22-Dual-STAR-T cell therapy (Hebei Yanda Ludaopei Hospital) | Phase 1 Clinical | Hebei Yanda Ludaopei Hospital | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details | |
| Anti-CD19/CD22 CAR-T cell therapy (The First Affiliated Hospital Of Soochow University) | Phase 2 Clinical | The First Affiliated Hospital Of Soochow University | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Human CD19 Targeted DASH CAR-T Cells (Hrain Biotechnology) | Phase 1 Clinical | Hrain Biotechnology Co Ltd | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| ET190L1-ARTEMIS T cells (Eureka Therapeutics) | ET190L1-ARTEMI; ET-190; ET190L1; ET190 | Phase 1 Clinical | Eureka Therapeutics Inc | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| GB5005 chimeric antigen receptor T cell therapy (Genbase) | GB-5005 | Phase 1 Clinical | Shanghai Genbase Biotechnology Co Ltd, Shanghai Genechem Co Ltd | Leukemia, B-Cell | Details |
| Englumafusp alfa | RG-6076; RO-7227166 | Phase 1 Clinical | F. Hoffmann-La Roche Ltd | Lymphoma, Non-Hodgkin | Details |
| SNUH-CD19-CAR-T Cell Therapy | SNUH-CD19-CAR-T | Phase 1 Clinical | Seoul National University Hospital | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| B-019 | B-019 | Phase 1 Clinical | Shanghai Pharmaceuticals Holding Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| MC-19PD1 CAR-T cell therapy (Peking University) | Phase 1 Clinical | Peking University | Lymphoma | Details | |
| CD19-7×19 CAR-T(Zhejiang University) | Phase 1 Clinical | Zhejiang University | Lymphoma, Large B-Cell, Diffuse | Details | |
| BRL-201 | BRL-201; PD1-19bbz; PD1-19bbz CAR-T | Phase 2 Clinical | Leukemia; Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| Anti CD19 chimeric antigen receptor T cell therapy (YaKe Biotechnology) | Phase 2 Clinical | Shanghai YaKe Biotechnology Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CD19/CD22 dual-target CAR-T (Yake biotechnology) | Phase 2 Clinical | Shanghai YaKe Biotechnology Co Ltd | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CN-201 | CN-201 | Phase 2 Clinical | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| Anbalcabtagene autoleucel | CRC-01 | Phase 2 Clinical | Curocell Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular | Details |
| CD19/CD20 dual-target CAR-T cell therapy (Shenzhen University General Hospital) | Phase 2 Clinical | Shenzhen University General Hospital | Lymphoma, B-Cell | Details | |
| QN-019a | QN-019a | Phase 1 Clinical | Hangzhou Qihan Biotechnology Co Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details |
| Bispecific CD19/22 CAR T cells | Phase 2 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| Anti-CD19 CAR CIK-cell therapy (Formula Pharmaceuticals) | CARCIK-CD19; CIK-CAR.CD19 | Phase 2 Clinical | Fondazione Matilde Tettamanti Menotti De Marchi Onlus | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| CNTY-101 | CNTY-101 | Phase 1 Clinical | Century Therapeutics Llc | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details |
| TI-1007 | TI-1007 | Phase 1 Clinical | Tianjin Timmune Biotech Inc | Lymphoma, B-Cell | Details |
| EB-103 | EB-103 | Phase 2 Clinical | Estrella Biopharma Inc | Lymphoma, B-Cell | Details |
| BinD-19 (Shenzhen BinDeBio) | BinD-19 | Phase 2 Clinical | Shenzhen Bindebio Ltd | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| CD19/CD22 Bispecific CAR-T Cell Therapy(Beijing Tongren Hospital) | Phase 2 Clinical | Beijing Tongren Hospital | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Recombinant humanized anti-CD19/CD3 bispecific antibody(New Time Pharmaceutical) | LNF-1904 | Phase 1 Clinical | Shandong New Time Pharmaceutical Co Ltd | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| Anti-CD19 CAR-T cell therapy (Juno Therapeutics) | Phase 1 Clinical | Juno Therapeutics Inc | Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| BZ019 | BZ-019 | Phase 2 Clinical | Shanghai Cell Therapy Group Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Primary mediastinal B cell lymphoma; Lymphoma, Follicular; Lymphoma | Details |
| CD19/CD20 Dual CAR-T Cell Therapy (China Immunotech) | HX-s001; YTS101; HX-s001/YTS101; HXYT-001 | Phase 1 Clinical | Beijing Qingyi Taike Pharmaceutical Technology Co Ltd, China Immunotech Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| AT-101 (AbClon) | AT-101 | Phase 2 Clinical | Abclon Inc | Hematologic Neoplasms; Lymphoma, Non-Hodgkin | Details |
| Dual CD19/CD20 targeting CAR-T therapy(Poseida Therapeutics) | Phase 1 Clinical | Poseida Therapeutics Inc | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| ALLO-501 | ALLO-501 | Phase 2 Clinical | Cellectis Sa | Lymphoma, B-Cell; Lymphoma, Follicular | Details |
| ALLO-501A | ALLO-501A | Phase 2 Clinical | Allogene Therapeutics Inc | Lymphoma, B-Cell | Details |
| ALETA-001 | ALETA-001 | Phase 2 Clinical | Aleta BioTherapeutics Inc | Lymphoma, B-Cell; Leukemia; Hematologic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin | Details |
| CD19/70 Bi-specific CAR-T Cell Therapy (Shenzhen Geno-Immune Medical Institute) | Phase 2 Clinical | Shenzhen Geno-Immune Medical Institute | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| CD19- and CD22 specific CAR (Seattle Children's Hospital) | Phase 1 Clinical | Seattle Children'S Hospital | Leukemia; Lymphoma | Details | |
| U-01 | U-01 | Phase 1 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Leukemia; Leukemia, B-Cell | Details |
| Azercabtagene zapreleucel | PBCAR-0191 | Phase 2 Clinical | Baxalta Incorporated | Hematologic Neoplasms; Leukemia, Lymphoid; Lymphoma, Non-Hodgkin | Details |
| CARTHIAE-1 | CARTHIAE-1 | Phase 1 Clinical | Miltenyi Biotec Inc, Hospital Israelita Albert Einstein | Lymphoma, B-Cell; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| GC019F | Phase 2 Clinical | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details | ||
| Budoprutug | Phase 2 Clinical | ValenzaBio Inc | Nephrosis, Lipoid; Glomerulonephritis, Membranous; Glomerulosclerosis, Focal Segmental | Details | |
| CD19/CD20 bispecific CAR-T cells (Henan Cancer Hospital) | Phase 1 Clinical | Henan Provincial Cancer Hospital | Lymphoma, B-Cell | Details | |
| BCMA-CD19 CAR T cell therapy (Hunan Siweikang Pharma) | Phase 1 Clinical | Hunan Siweikang Pharmaceutical Co Ltd | Multiple Myeloma | Details | |
| U-CART (Shanghai Bioray Laboratory Inc) | Phase 2 Clinical | Hematologic Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | ||
| UCART-19 | S-68587; UCART-19 | Phase 1 Clinical | Cellectis Sa | Leukemia, Lymphoid; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| GC-502 | GC-502 | Phase 1 Clinical | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| Anti-CD19 and anti-CD22 CAR T cell therapy (Hebei Senlang Biotechnology) | Phase 1 Clinical | Lymphoma, B-Cell; Leukemia, B-Cell | Details | ||
| Anti-CD19 CAR-T cell therapy (The First Affiliated Hospital of Nanchang University) | Phase 2 Clinical | The First Affiliated Hospital Of Nanchang University | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma | Details | |
| Anti-CD19 CAR-T cell therapy (Sinobioway) | Phase 2 Clinical | Sinobioway Biomedicine Co Ltd | Lymphoma, Large B-Cell, Diffuse; Lymphoma | Details | |
| Anti-CD19 CAR-T cell therapy (iCarTAB BioMed) | Phase 1 Clinical | Aikangd Biomedical Technology (Suzhou) Co Ltd | Leukemia; Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| Humanized CD19 CAR-T cell therapy (Yake) | YK-C19 | Phase 1 Clinical | Shanghai YaKe Biotechnology Co Ltd | Lymphoma, B-Cell; Leukemia, Lymphoid; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Murine CD19 CAR-T cell therapy (Yake) | Phase 1 Clinical | Shanghai YaKe Biotechnology Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| CD19/CD22 dual-target CAR-T cell therapy (Shenzhen University General Hospital) | Phase 2 Clinical | Shenzhen University General Hospital | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Burkitt Lymphoma | Details | |
| Anti-CD19 and anti-CD20 CAR-T cell therapy (Medical College of Wisconsin) | Phase 2 Clinical | The Medical College Of Wisconsin Nonprofit | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Burkitt Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma | Details | |
| CD19 UCAR-T cell therapy (Shanghai Longyao Biotechnology) | Phase 1 Clinical | Shanghai Longyao Biological Technology Co Ltd | Leukemia, Myeloid, Acute | Details | |
| CD19-TriCAR-T/SILK Cell Therapy(Timmune Biotech) | Phase 1 Clinical | Tianjin Timmune Biotech Inc | Leukemia; Lymphoma, Non-Hodgkin | Details | |
| Vadacabtagene leraleucel | 19-28z CAR T cells; 19-28z+ T cells; JCAR-015; JCAR-15; 19-28z-T-Cells | Phase 1 Clinical | Juno Therapeutics Inc, Memorial Sloan Kettering Cancer Center, Seattle Children'S Research Institute, Fred Hutchinson Cancer Research Center | Leukemia; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| ET-019002 | ET-019002 | Phase 1 Clinical | Eureka Therapeutics Inc | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| FT-596 | FT-596 | Phase 1 Clinical | Fate Therapeutics Inc | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| CC-97540 | CC-97540 | Phase 1 Clinical | Juno Therapeutics Inc | Lymphoma, Large B-Cell, Diffuse; Lupus Erythematosus, Systemic; Lymphoma, Non-Hodgkin; Lymphoma | Details |
| Humanized CAR19T2 T Cell therapy(Zhaotai Cell Bio-tech) | Phase 2 Clinical | Guangdong Zhaotai Cell Bio-tech Co Ltd | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CAR-T-19 Cell Therapy (The General Hospital Of The People'S Liberation Army) | Phase 1 Clinical | Pla General Hospital | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details | |
| CD19 PD-1/CD28 CAR-T Cell Therapy (Second Affiliated Hospital School Of Zhejiang University School Of Medicine) | Phase 1 Clinical | Second Affiliated Hospital Of Zhejiang University School Of Medicine | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Follicular; Primary mediastinal B cell lymphoma | Details | |
| CD19-CART (IL-6 secretion knockdown, Unicar-Therapy Bio-medicine Technology) | ssCART-19 | Phase 1 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell | Details |
| Humanized anti-CD19 and anti-CD20 dual specific CAR-T cell therapy (Fujian Medical University) | Phase 1 Clinical | Fujian Medical University | Lymphoma, Large B-Cell, Diffuse | Details | |
| Anti-CD19+/BCMA CAR-T cells (Hrain Biotechnology) | Phase 1 Clinical | Hrain Biotechnology Co Ltd | POEMS Syndrome | Details | |
| CLBR-001/SWI-019 | CLBR-001/SWI-019 | Phase 1 Clinical | Abbvie Inc, California Institute For Biomedical Research | Lymphoma, B-Cell | Details |
| ICT19G1 | ICT-19G1 | Phase 1 Clinical | Innovative Cellular Therapeutics Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti-CD19 and anti-CD22 CAR-T cell therapy(IASO Biotherapeutics) | CT-120 | Phase 2 Clinical | Nanjing Iaso Biotherapeutics Co Ltd | Lymphoma, B-Cell; Leukemia, B-Cell; Lymphoma, Non-Hodgkin | Details |
| CAR-DC(Zhejiang University) | Phase 1 Clinical | Zhejiang University | Lymphoma, B-Cell | Details | |
| Anti-CD19 CAR-T cells with TNFRS19 transmembrane domain | Phase 2 Clinical | Federal Research Institute of Pediatric Hematology Oncology and Immunology | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| PBCAR19B | PBCAR-19B | Phase 1 Clinical | Baxalta Incorporated, Precision Biosciences Inc | Hematologic Neoplasms; Neoplasms | Details |
| IMPT-514 | IMPT-514 | Phase 2 Clinical | ImmPACT Bio USA Inc | Lupus Nephritis; Lupus Erythematosus, Systemic | Details |
| Anti-CD19 single-stranded antibody CAR T-cell therapy (Beijing Yongtai Rec Biotechnology) | Phase 1 Clinical | Beijing Yongtai Rec Biotechnology Co Ltd | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CD19-Car T Cell Therapy(City Of Hope National Medical Center) | Phase 1 Clinical | City Of Hope National Medical Center, National Cancer Institute | Lymphoma, B-Cell; Leukemia, Hairy Cell; Lymphomatoid Granulomatosis; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma | Details | |
| CD19-UCART | BRL-301 | Phase 1 Clinical | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| HY-004 (Juventas) | HY-004 (Juventas) | Phase 1 Clinical | Juventas Cell Therapy Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| CD19 CAR-T cell therapy (National University of Malaysia) | Phase 3 Clinical | National University Of Malaysia | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| 19273-4SCAR | 19273-4SCAR | Phase 2 Clinical | Peking University | Lymphoma, B-Cell | Details |
| 19(T2)28z1xx Chimeric Antigen Receptor (CAR) Modified T Cells | TAK-940 | Phase 1 Clinical | Takeda, Memorial Sloan Kettering Cancer Center | Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Leukemia; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Primary mediastinal B cell lymphoma; Lymphoma, Follicular; Waldenstrom Macroglobulinemia; Burkitt Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell; Central Nervous System Lymphoma | Details |
| GNR-084 | GNR-084 | Phase 2 Clinical | Generium Pharmaceuticals, Iontas | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Allogeneic EBV-specific anti-CD19 CAR-T cell therapy (Memorial Sloan-Kettering Cancer Center) | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| CART-19 cells (Henan Hualong Biotechnology) | Phase 2 Clinical | Henan Hualong Biotechnology | Leukemia, Lymphoid | Details | |
| SYNCAR-001 | SYNCAR-001 | Phase 1 Clinical | Synthekine Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Lymphoma, Follicular; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Senl-1904A | Senl-1904A; Senl_1904A | Phase 1 Clinical | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CAR-T 19 cell therapy (University of Pennsylvania) | Phase 2 Clinical | University Of Pennsylvania | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CD19-CAR-NK(JD Biotech) | JD-010; JD010 | Phase 1 Clinical | Beijing JD Biotech Co Ltd | Hematologic Neoplasms | Details |
| GLPG-5101 | 19-CP-02; GLPG5101; 19CP02 | Phase 2 Clinical | CellPoint BV | Lymphoma, Non-Hodgkin | Details |
| KT-030 | KT-030 | Phase 1 Clinical | Nanjing Kati Medical Technology Co Ltd | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| IMPT-314 | IMPT-314 | Phase 2 Clinical | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| CAR-NK-CD19 Cell Therapy (Wuhan Union Hospital) | Phase 1 Clinical | Wuhan Union Hospital | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| CD19-CAR-T cell therapy (China Immunotech/Avalon Globocare) | AVA-001 (Avalon GloboCare); AVA-001 | Phase 1 Clinical | China Immunotech Co Ltd, Avalon Globocare Corp | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| Chimeric antigen receptor T cell therapeutics (targeted CD19/CD20/CD22/CD30,Shanghai Unicar-Therapy Bio-medicine) | Phase 2 Clinical | Shanghai Unicar-Therapy Bio-Medicine Technology Co Ltd | Pancreatic Neoplasms; Lymphoma, Non-Hodgkin | Details | |
| Anti-CD19 chimeric antigen receptor T cell therapy (Immune cell) | Phase 1 Clinical | Immune Cell Inc | Lymphoma, B-Cell; Leukemia, B-Cell | Details | |
| RGV-004 | RGV-004 | Phase 1 Clinical | Hangzhou Rongu Biotechnology Co Ltd | Lymphoma, B-Cell | Details |
| CAR-NK019(Second Affiliated Hospital, School of Medicine, Zhejiang University) | Phase 1 Clinical | Second Affiliated Hospital School Of Zhejiang University School Of Medicine | Lymphoma, B-Cell; Lymphoma, Non-Hodgkin | Details | |
| Denocabtagene Ciloleucel(Beijing Yongtai Ruike Biotechnology Company) | RC19D2; CAR-T-19-D2; RC19-D2; CAR-T-19-DNR | Phase 2 Clinical | Beijing Yongtai Rec Biotechnology Co Ltd | Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| KITE-363 | KITE-363 | Phase 1 Clinical | Kite Pharma | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse | Details |
| 1-A-46 | BR-110; 1A46; 1-A-46; CMG1A46; CMG1A-46; BR110 | Phase 2 Clinical | Chengdu Chimagen Biosciences Co Ltd, Hisun Biopharmaceutical Co Ltd, BioRay Pharmaceutical Co Ltd | Lymphoma, B-Cell; Neoplasms; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details |
| CTL-119 | CTL-119 | Phase 1 Clinical | Novartis Pharma Ag, University Of Pennsylvania | Multiple Myeloma | Details |
| KUR-502 CD19 CAR-NKT | CMD-502; KUR-502 | Phase 1 Clinical | Athenex Inc | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| TAK-007 | TAK-007 | Phase 2 Clinical | The University Of Texas MD Anderson Cancer Center | Lymphoma, Non-Hodgkin | Details |
| CC-312 | CC-312 | Phase 1 Clinical | CytoCares (Shanghai) Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| ET-02 | ET-02 | Clinical | Edigene Inc | Lymphoma, B-Cell | Details |
| Anti-CD19 CAR-T cell therapy (Xuzhou Medical University) | Phase 2 Clinical | Xuzhou Medical University (Xzmu) | Lymphoma, Non-Hodgkin | Details | |
| CD19-CD34 CAR transduced T cell therapy (Loyola University) | Phase 1 Clinical | Loyola University Chicago | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Autologous CD19 CAR+ EGFTt + T cells (Seattle Children's Hospital) | Phase 1 Clinical | Seattle Children'S Hospital | Leukemia; Leukemia, B-Cell | Details | |
| Zeripatamig | NI-1701; TG-1801 | Phase 1 Clinical | Novimmune Sa | Lymphoma, B-Cell | Details |
| Emfizatamab | GNC-038 | Phase 2 Clinical | Sichuan Baili Pharmaceutical Co Ltd | Solid tumours; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Extranodal NK-T-Cell; Lymphoma, Non-Hodgkin; Lymphoma, T-Cell; Central Nervous System Lymphoma | Details |
| GC-012F | GC-012F; GC012F | Phase 2 Clinical | Multiple Myeloma; Lupus Erythematosus, Systemic; Lymphoma, Non-Hodgkin | Details | |
| Anti-CD19 CAR-T cell therapy (Zhongyuan Union Cell & Gene Engineering) | Phase 1 Clinical | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | ||
| Anti-CD19 CAR T-cell therapy (Dana-Farber Cancer Institute/Memorial Sloan-Kettering Cancer Center/Boston Children's Hospital) | Phase 2 Clinical | Memorial Sloan Kettering Cancer Center, Dana-Farber Cancer Institute, Boston Children'S Hospital | Leukemia | Details | |
| CAR-iNKT cells therapy (North Jiangsu People's Hospital) | Phase 1 Clinical | Affiliated Hospital Of Jiangsu University | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Allogeneic CD-19 CAR-T Cell Therapy | Phase 2 Clinical | Shenzhen University General Hospital | Leukemia; Lymphoma, B-Cell | Details | |
| Anti-CD19-CAR γδT cells (Beijing Doing Biomedical) | Phase 1 Clinical | Beijing Doing Biomedical Co Ltd | Leukemia; Lymphoma | Details | |
| AUTO-3 | AUTO-3 | Phase 2 Clinical | University College London | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti-CD19 chimeric antigen receptor T cell therapy (Innovative Cellular Therapeutics) | Phase 2 Clinical | Zhejiang University, Innovative Cellular Therapeutics Co Ltd, Kunming Medical University | Lymphoma, B-Cell; Myasthenia Gravis; Lymphoma, Large B-Cell, Diffuse; Neuromyelitis Optica; Leukemia, B-Cell; Lymphoma | Details | |
| BCM-5011 | BCM-5011 | Clinical | ImmuneCyte Inc | Lymphoma, B-Cell | Details |
| LCAR-AIO | LCAR-AIO; VHH CAR-T | Phase 1 Clinical | Nanjing Legend Biotechnology Co Ltd | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| Anti-CD19 and Anti-CD20 Bicistronic Chimeric Antigen Receptor T Cells(NIH) | Phase 1 Clinical | National Cancer Institute | Lymphoma, B-Cell; Leukemia, Lymphocytic, Chronic, B-Cell | Details | |
| Anti-CD19 CAR-T Cells (Yan'an Affiliated Hospital of Kunming Medical University) | Phase 1 Clinical | Kunming Medical University | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Autologous CAR19 T lymphocytes Therapy | Phase 1 Clinical | Institute of Hematology and Blood Transfusion, Czech Republic | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| YK-012 | YK012 | Phase 1 Clinical | Yikesite (Beijing) Pharmaceutical Technology Development Co Ltd | Lymphoma, B-Cell | Details |
| UCART019 | UCART-019 | Phase 2 Clinical | Pla General Hospital | Lymphoma, B-Cell; Leukemia, B-Cell | Details |
| KD-019 (Nanjing KAEDI Biotech) | KD-C19 (Nanjing KAEDI Biotech) | Phase 1 Clinical | Nanjing Kaedi Biotechnology Co Ltd | Leukemia; Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details |
| PIT-565 | PIT-565 | Phase 1 Clinical | Novartis Pharma Ag | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Myeloid, Acute; Lymphoma, Non-Hodgkin | Details |
| Autologous CD19-directed chimeric antigen receptor (CAR) T-cell therapy (University of Alberta) | Phase 2 Clinical | University Of Alberta | Precursor Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Non-Hodgkin | Details | |
| FT-819 | FT-8198; FT-819; FT 8198 | Phase 1 Clinical | Memorial Sloan Kettering Cancer Center | Lymphoma, B-Cell; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| Anti-CD19/CD22 CAR-T cell therapy (Jiao Tong University) | Phase 1 Clinical | Shanghai Jiaotong University | Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Allogeneic anti-CD19 CAR T-cell therapy(EdiGene/Peking University/Chinese PLA Hospital) | Peking University, Pla General Hospital, Edigene Inc | Details | |||
| iPD1 CD19 eCAR-T therapy (Marino Biotechnology) | Phase 1 Clinical | Chengdu Yinhe Biomedicine Co Ltd, Beijing Marino Biotechnology Co Ltd | Lymphoma, B-Cell; Glioblastoma | Details | |
| NKX-019 | NKX-019 | Phase 1 Clinical | Nkarta Inc | Hematologic Neoplasms; Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Lymphoma, Mantle-Cell; Lymphoma; Lymphoma, Non-Hodgkin; Lymphoma, Large-Cell, Anaplastic; Waldenstrom Macroglobulinemia; Leukemia, Lymphocytic, Chronic, B-Cell | Details |
| CD19-CAR-DNT cells Therapy(Zhejiang Ruijiamei Biotechnologies Co Ltd) | RJMty-19 | Phase 1 Clinical | Zhejiang Ruijiamei Biotechnologies Co Ltd | Lymphoma, B-Cell; Autoimmune Diseases; Lupus Erythematosus, Systemic | Details |
| Anti-CD7 and anti-CD19 chimeric antigen receptor T cell therapy | GC-197 | Phase 2 Clinical | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| Anti-CD19-CAR CMV-specific T-lymphocytes cell therapy (City Of Hope National Medical Center) | Phase 1 Clinical | City Of Hope National Medical Center | Lymphoma, B-Cell; Lymphoma, Large B-Cell, Diffuse; Lymphoma, Mantle-Cell; Lymphoma, Non-Hodgkin | Details | |
| CAR-T-19(Immunotech Biopharm) | CAR-T-19(Immunotech Biopharm) | Phase 2 Clinical | Immunotech Biopharm Ltd | Lymphoma, B-Cell; Precursor Cell Lymphoblastic Leukemia-Lymphoma; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Leukemia, B-Cell; Lymphoma | Details |
| Dual Anti-CD22/CD19 Chimeric Antigen Receptor-directed T Cells (CART2219.1) therapy (KK Women's and Children's Hospital) | Phase 2 Clinical | Kk Women'S And Children'S Hospital | Lymphoma, B-Cell; Leukemia, Lymphoid; Precursor B-Cell Lymphoblastic Leukemia-Lymphoma; Precursor Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| CD19 CAR engineered autologous T-cells Therapy(Sabz Biomedicals) | Phase 1 Clinical | Sabz Biomedicals | Leukemia, Biphenotypic, Acute | Details | |
| IASO-782 | IASO-782; IASO782 | Phase 1 Clinical | Shanghai Xunlu Biotechnology Co Ltd | Purpura, Thrombocytopenic, Idiopathic; Anemia, Hemolytic, Autoimmune; Thrombocytopenia; Anemia, Hemolytic | Details |
| Autologous hematopoietic stem cell therapy (YaKe Biotechnology) | Phase 1 Clinical | Zhejiang University, Ningbo Yake Biotechnology Co Ltd | Lymphoma | Details | |
| Allogeneic Second-generation CD19-CAR T Cells therapy(Bambino Gesu Hospital And Research Institute) | Phase 1 Clinical | Bambino Gesu Hospital And Research Institute | Precursor B-Cell Lymphoblastic Leukemia-Lymphoma | Details | |
| ATA-3219 | ATA-3219; ATA3219 | Phase 1 Clinical | Atara Biotherapeutics Inc | Lymphoma, Non-Hodgkin | Details |
This web search service is supported by Google Inc.








