
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































 Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order. Order Online! Now! Get your $50 coupon for online order.
Order Online! Now! Get your $50 coupon for online order.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
 Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!  Fill out organ-on-a-chip questionnaire to win a FREE gift!
Fill out organ-on-a-chip questionnaire to win a FREE gift!
> Insights > Extra Caution for safety and efficacy evaluation of COVID-19 vaccines According to the landscape documents of COVID-19 candidate vaccines prepared by the World Health Organization, there are 44 candidate vaccines in clinical trials globally, 10 of which are in Phase III (Table 1). Additionally, we have 154 candidate vaccines in the preclinical stage worldwide.
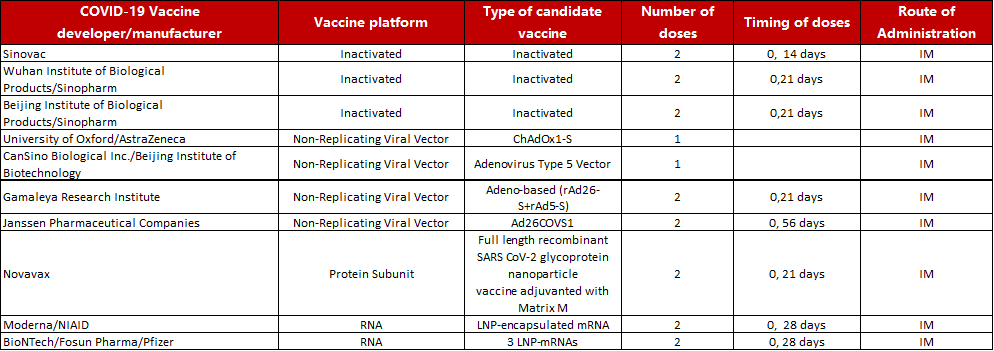
Table 1 Ten candidate vaccines in Phase III clinical trial
Source: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.
A typical development cycle for a vaccine is about 10 to 15 years. Given this COVID-19 public health emergency, scientists are working very hard to reduce the development cycle to a year or two. Due to the rush of vaccine development and the insufficient understanding of the vaccine's immune responses, extra caution could be used when evaluating the safety and efficacy of the vaccine. Two major studies of the COVID-19 vaccine from AstraZeneca and Johnson& Johnson, both paused because of potential safety concerns earlier. However, announced last Friday, both trials are set to restart.
Currently, clinical trials are being conducted according to the rigorous standards set forth by FDA in their June 2020 guidance document, Development and Licensure of Vaccines to Prevent COVID-19. If a vaccine meets its safety and effectiveness standards, it will be approved for use or authorized for emergency use. Continued vaccine safety monitoring after approval/ authorization can pick up on adverse events that may not have been seen in clinical trials. If an unexpected adverse event is seen, experts quickly study it further to assess whether it is a true safety concern. Experts then decide whether changes are needed in U.S. vaccine recommendations. This monitoring is critical to help ensure that the benefits continue to outweigh the risks for people who receive vaccines. [1]
There is still a long way to go for COVID-19 vaccine development and testing. ACROBiosytems, specialized in recombinant protein, developed a series of COVID-19-related protein products, including S trimer protein and the titer measurement kits to support vaccine development.
Application 1: Vaccine immunogenicity evaluation ----- IgG antibody measurement
Recommended product 1: SARS-CoV-2 S protein, His Tag, Super stable trimer (MALS & NS-EM verified)
Catalog Number: SPN-C52H9
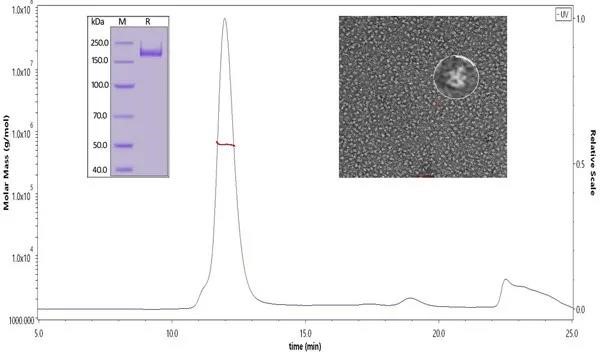
Fig. 1 ACRO SARS-CoV-2 S protein was verified through SDS-PAGE, SEC-MALS, and negative staining electron microscopy. Our S trimer protein has over 90% purity with an MW of 550-660 kDa. According to the data of negative staining electron microscopy, the S trimer protein has a similar size and appearance compared to references.
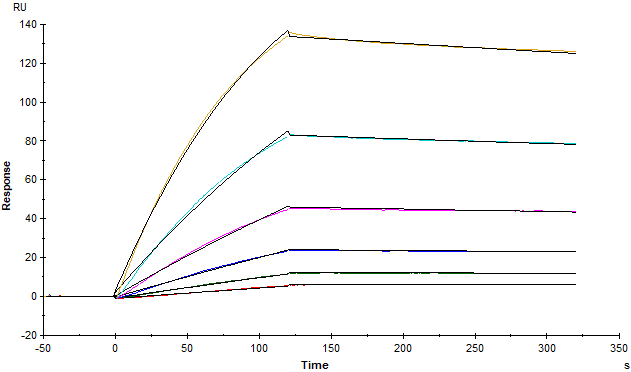
Fig. 2 The binding affinity of SARS-CoV-2 S trimer (Cat. No.SPN-C52H9) and human ACE2 (Cat. No. AC2-H5257) was evaluated using Biacore. The KD was 5.82nM.
Fig. 3 Antibody measurement in recovered patient samples using SARS-CoV-2 S trimer (Cat. No.SPN-C52H9) with a very good signal-noise ratio.
Recommended product 2: Anti-SARS-CoV-2 Antibody IgG Titer Serologic Assay kit (S protein trimer)
Catalog Number: TAS-K007
A rapid and effective assay kit for IgG antibody quantification in serum/plasma samples. This established method has advantages, including low background and a good signal to noise ratio.
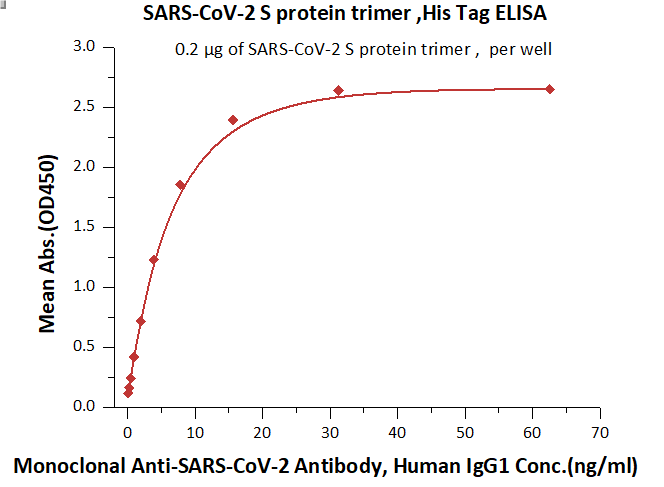
Fig. 4 Immobilized SARS-CoV-2 S protein at 2 μg/mL (100 μL/well) can bind Monoclonal Anti-SARS-CoV-2 Antibody, Human IgG1 in 1:50 human serum. Detection was performed using the HRP-Conjugated Anti-human IgG antibody with a sensitivity of 12ng/mL.
Recommended product 3: Anti-SARS-CoV-2 Antibody IgG Titer Serologic Assay kit (Spike protein RBD)
Catalog Number: TAS-K002
A rapid and effective assay kit for IgG antibody quantification in serum/plasma samples. This established method has advantages, including low background and a good signal to noise ratio.
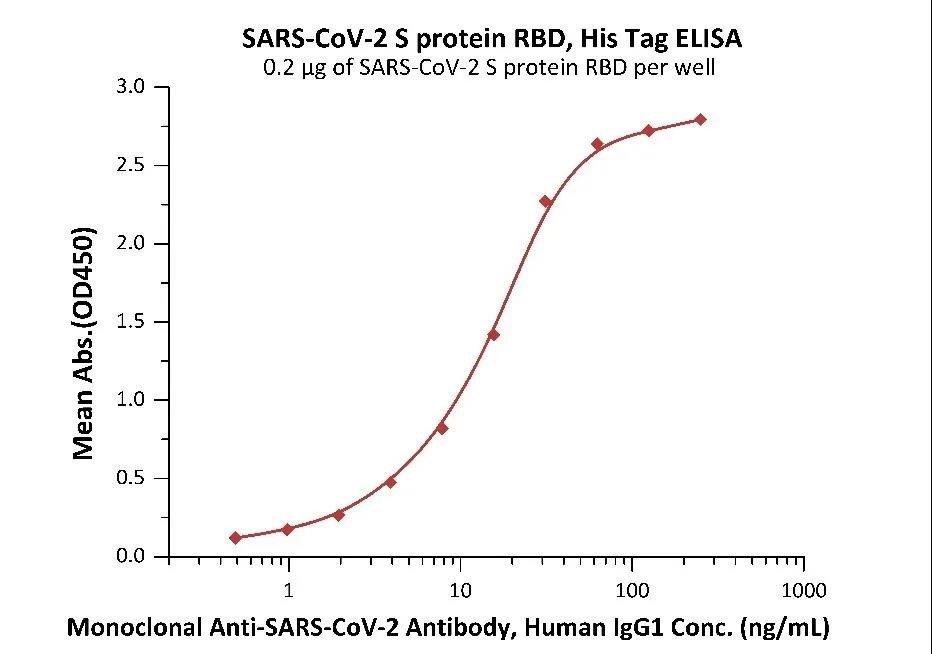
Fig. 5 Immobilized SARS-CoV-2 S protein RBD at 2 μg/mL (100 μL/well) can bind Monoclonal Anti-SARS-CoV-2 Antibody, Human IgG1 in 1:50 human serum. Detection was performed using the HRP-Conjugated Anti-human IgG antibody with a sensitivity of 24 ng/mL.
Application 2: Vaccine clinical immunogenicity evaluation ----- Neutralizing antibody measurement
Recommended product 1: Anti-SARS-CoV-2 Neutralizing Antibody Titer Serologic Assay kit
Catalog Number: TAS-K003
This kit is intended for serologic measurement for the titer of Anti-SARS-CoV-2 neutralizing antibody in serum/plasma samples.
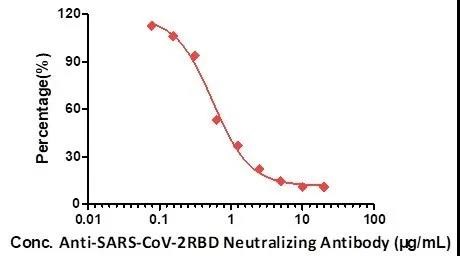
Fig. 6 Neutralizing titer of Anti-SARS-CoV-2 Neutralizing Antibody, Human IgG1 (Cat.No.SAD-S35) measured by Anti-SARS-CoV-2 neutralizing antibody titer serologic assay kit (Cat. No. TAS-K003).
Recommended product 2: Anti-SARS-CoV-2 RBD Potent Neutralizing Antibody, Chimeric mAb, Human IgG1 (AM128)
Catalog Number: SPD-M128
This neutralizing antibody was verified using a pseudotyped virus. It is designed as a standard reference for the vaccine evaluation assays.
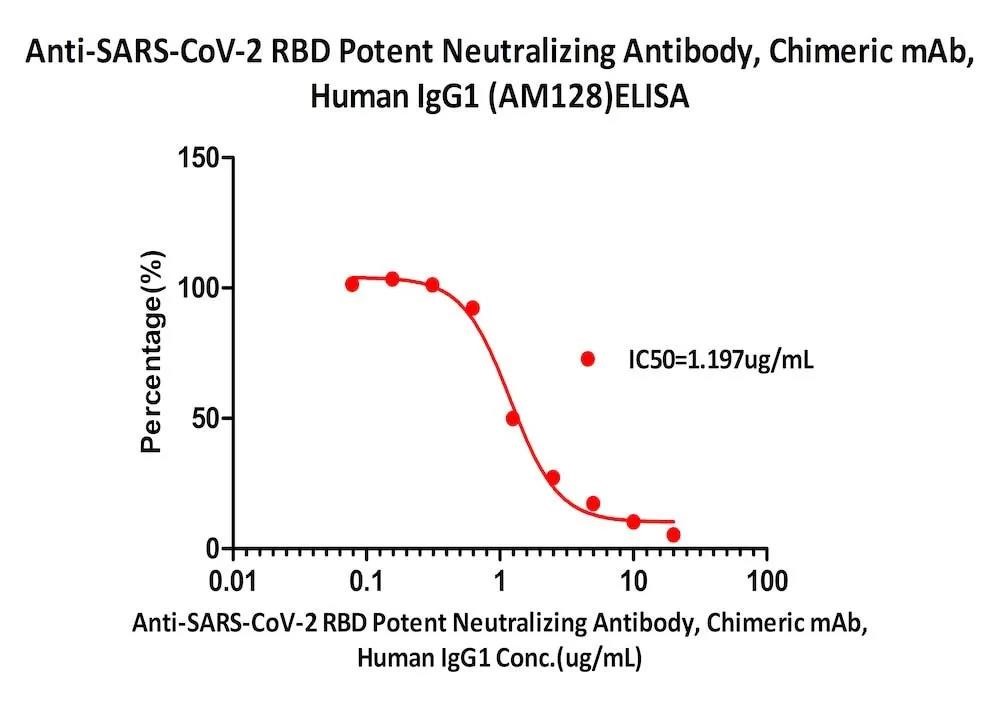
Fig. 7 Serial dilutions of Anti-SARS-CoV-2 RBD Potent Neutralizing Antibody, Chimeric mAb, Human IgG1 (AM128) (Cat. No. SPD-M128) was detected by SARS-CoV-2 Inhibitor screening Kit (Cat. No. EP-105) with a half maximal inhibitory concentration (IC50) of 1.197 μg/mL.
Application 3: Vaccine post-market evaluation ----- Neutralizing antibody measurement
Recommended products:
Catalog number | Products | Application |
SPD-C52H1 | SARS-CoV-2 (COVID-19) S protein RBD, His Tag | Lateral Flow Assay |
AC2-H52H8 | Human ACE2 /ACEH Protein, His Tag (MALS verified) |
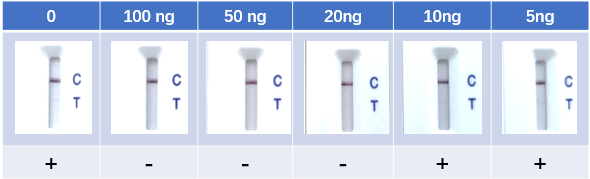
Fig. 8 Lateral Flow Assay with spike-in neutralizing antibody serum samples (Cat No. SPD-M128). The anti-mouse IgG and RBD-his protein were coated to the control line and test line respectively. Colloidal gold labeled ACE2 and mouse IgG were immobilized in conjugate release pad.
Additionally, ACRO developed an antibody isotyping kit, which can be used for anti-SARS-CoV-2 IgG1, IgG2, IgG3 and IgG4 testing. There will be more validated kits available for COVID-19 studies.
Reference:
1. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety.html
This web search service is supported by Google Inc.







