> testAAAA 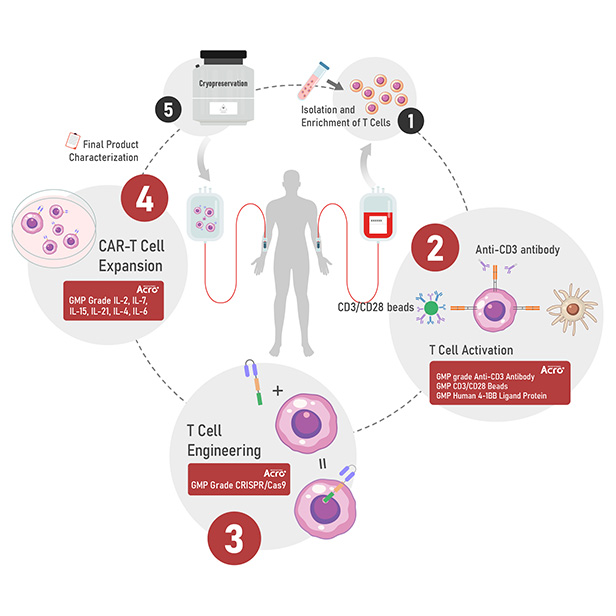
 Efficiently activate and expand T cells
Efficiently activate and expand T cells Adheres to GMP grade quality system
Adheres to GMP grade quality system Animal-free materials for enhanced safety
Animal-free materials for enhanced safety Rigorous viral removal steps and testing
Rigorous viral removal steps and testing High stability; high batch-to-batch consistency
High stability; high batch-to-batch consistency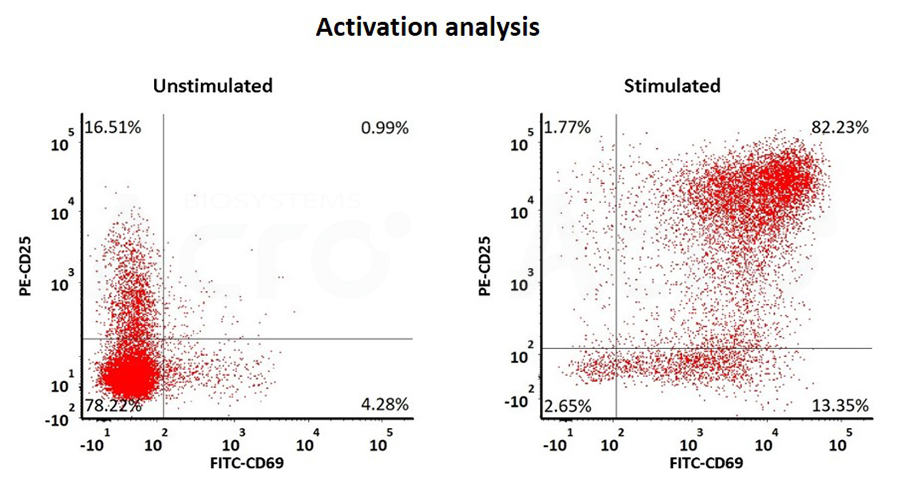
Figure 1. The human T cells were stimulated with GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads (Cat. No. GMP-MBS001) for 24hrs, and the activation was assessed by measuring expression of both activation markers CD25 and CD69 expression on the T cells surface by stanning with PE labeled anti-human CD25 antibody and FITC labeled anti-human CD69 antibody respectively (QC tested).
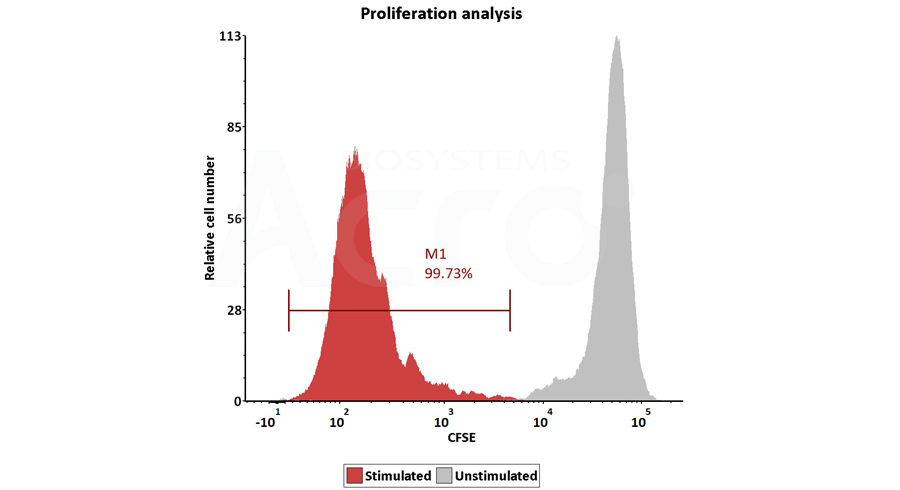
Figure 2. The human T cells were labeled with carboxy fluorescein succinimidyl ester (CFSE) and stimulated with GMP ActiveMax® Human T cell Activation/Expansion CD3/CD28 Beads (Cat. No. GMP-MBS001), and then the proliferation of the T cells was assessed with CFSE dilution assay by flow cytometry on day 5 after stimulation (QC tested).
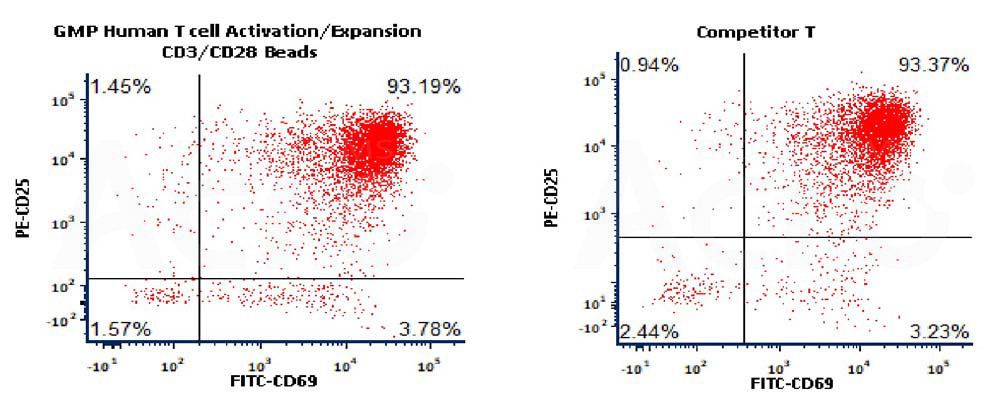
Figure 3. Activation of the purified human T Cells. The purified human T cells were activated using Human T cell Activation/Expansion CD3/CD28 Beads, (Cat. No. GMP-MBS001) and Competitor-Beads respectively for 24 hours with CTS Optimizer Medium. Cells were fluorescently stained using PE labeled anti-human CD25 antibody and labeled FITC anti-human CD69 antibody and analyzed by flow cytometry.
CAR-T
TCR-T

TIL
Treg
After activation, T cells also rely on various cytokines (IL-2, IL-7, IL-15, IL-21, and TNF- α, etc.) to further promote proliferation and activation.
 Comprehensive quality release verification, with 16 quality control indicators
Comprehensive quality release verification, with 16 quality control indicators Enhanced safety (aseptic mycoplasma, exogenous virus, residual endotoxin testing)
Enhanced safety (aseptic mycoplasma, exogenous virus, residual endotoxin testing) Pharmaceutical-grade production facility
Pharmaceutical-grade production facility Support for online and offline audits
Support for online and offline audits Completion of FDA Drug Master File (DMF) registration
Completion of FDA Drug Master File (DMF) registration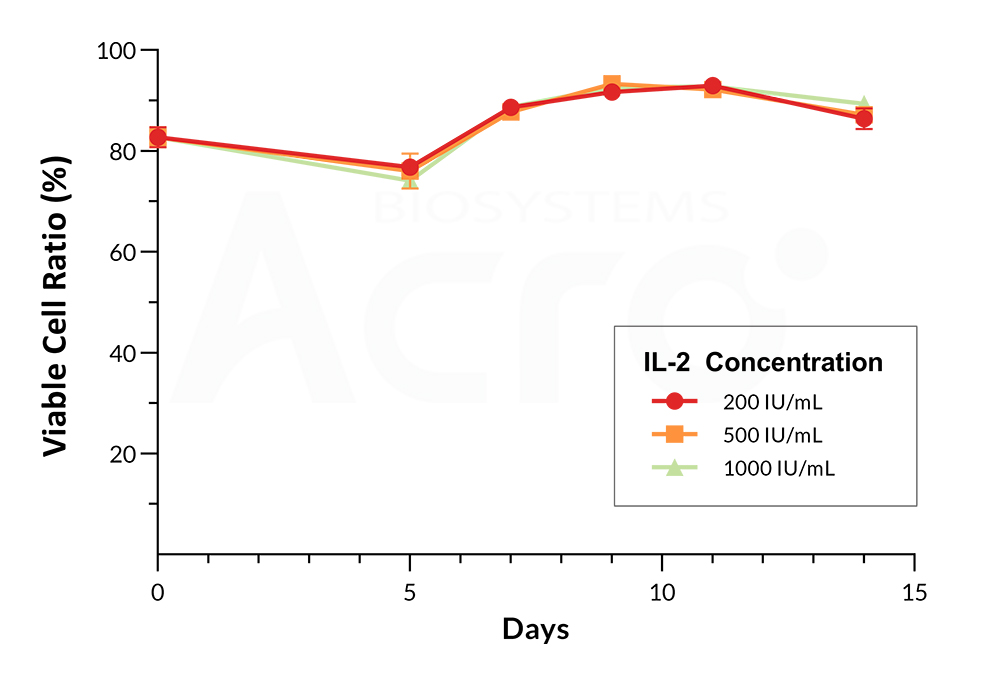
Figure 1. Cell growth curves of PBMCs cultured with IL-2. Varying concentrations of GMP IL-2 (Cat. No. GMP-L02H14) were added as a growth factor supplement to PBMCs revealing optimal cell expansion using a concentration of 500 IU/mL at Day 14.
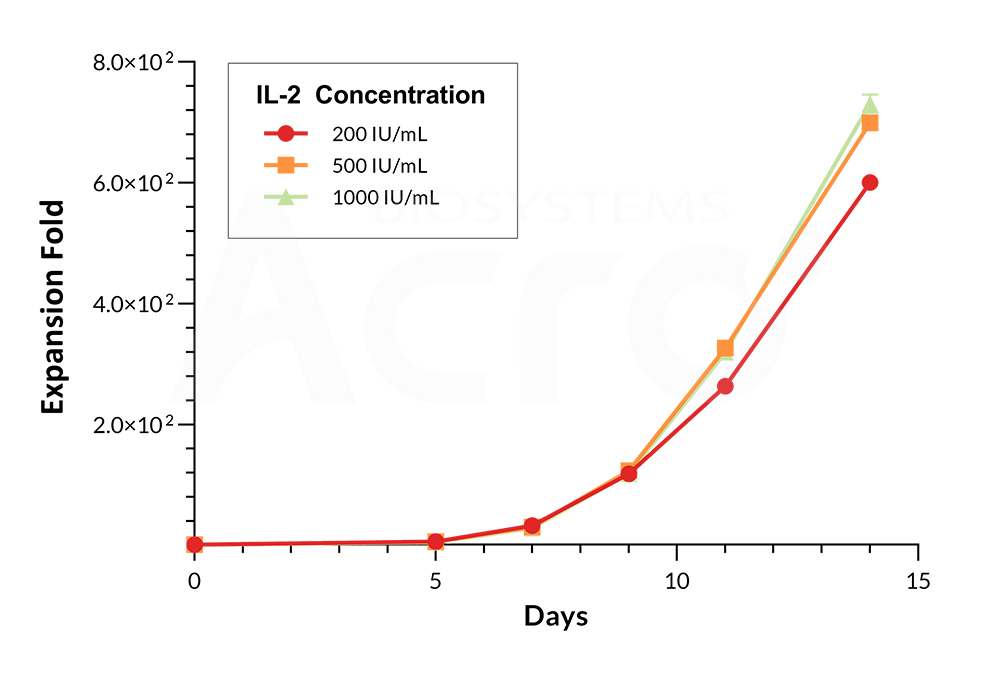
Figure 2. Cell viability curve of PBMCs cultured with IL-2. Increasing concentrations of GMP IL-2 (Cat. No. GMP-L02H14) were added as a growth factor supplement to PBMCs showing consistent and high cell viabilities up to Day 14.
 High purity, high enzyme activity, high cleavage efficiency
High purity, high enzyme activity, high cleavage efficiency Possesses nuclear localization signals to enhance editing efficiency
Possesses nuclear localization signals to enhance editing efficiency Aseptic, ultra-low endotoxin
Aseptic, ultra-low endotoxin Produced in GMP-compliant facilities and undergoes QC testing
Produced in GMP-compliant facilities and undergoes QC testing High biological activity verified by in vitro and in vivo enzyme activity, high TCR knockout activity.
High biological activity verified by in vitro and in vivo enzyme activity, high TCR knockout activity.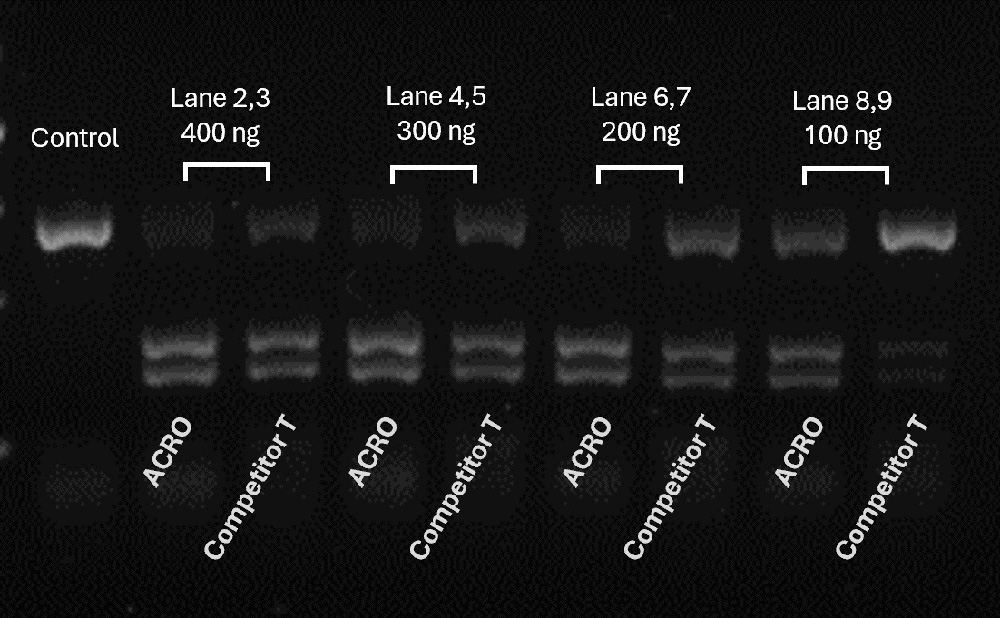
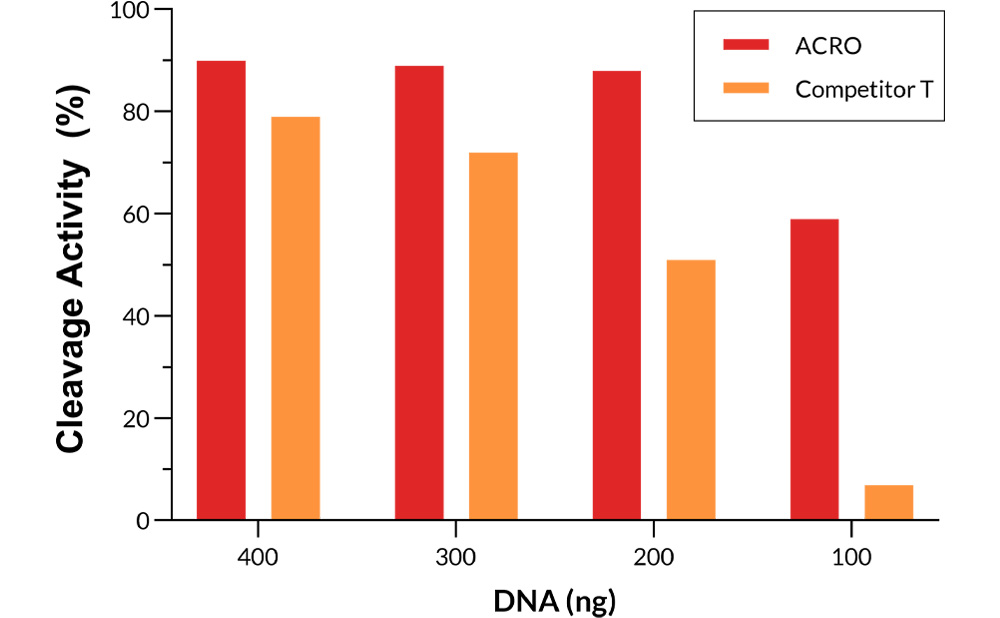
Different amounts of Cas9 were incubated with the same amount of excess gRNA and plasmid for 60 minutes at 37°C. When using 400-200 ng Acro Cas9, the cutting efficiency is greater than 90%. In comparison, when using a 200 ng Competitor T, the cutting efficiency is only about 50%.
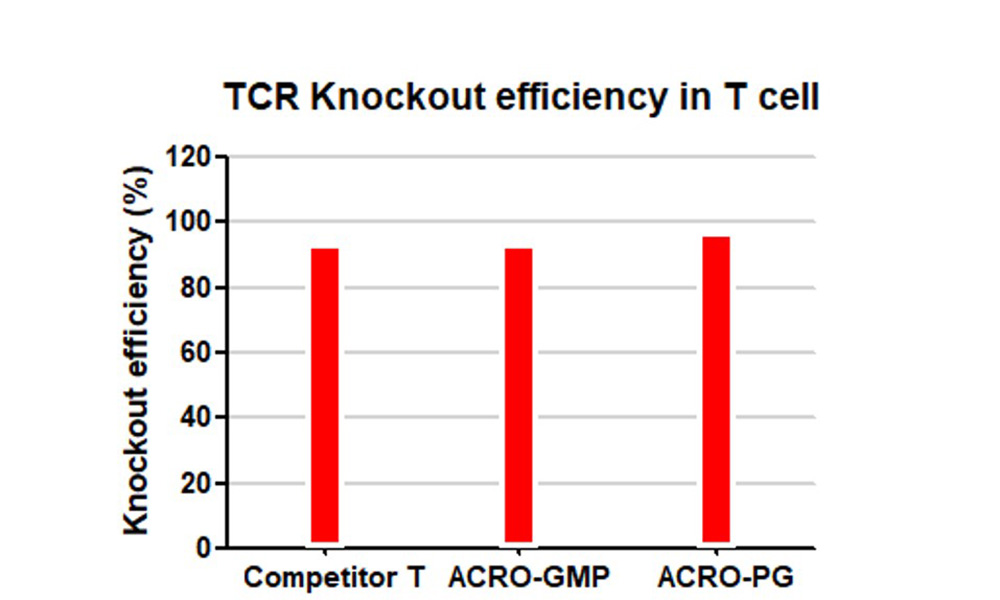
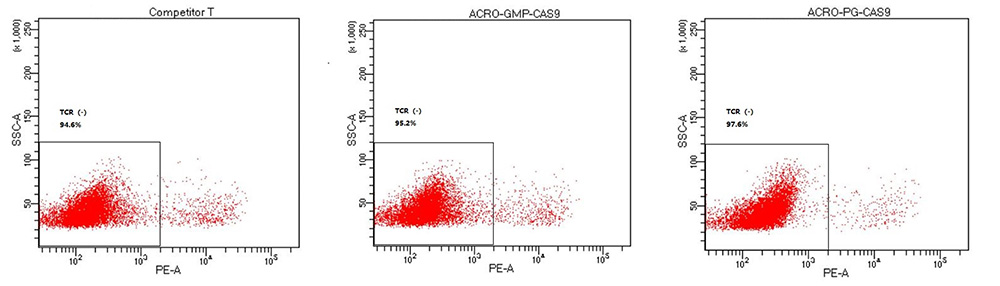
The TCR knockout efficiency with GMP GENPower™ NLS-Cas9 Nuclease in human primary T cells, GMP GENPower™ NLS-Cas9 Nuclease achieved over 95% knockout efficiency.

The production of lentiviral packaging plasmids is a critical step in CAR-T cell preparation. During this process, nucleic acid residues can be generated including host residual nucleic acid, poses significant safety concerns due to potential infectious or tumorigenic risks.
Our enzyme modification technology platform, coupled with collaborative AI simulation and high-throughput screening, helped us independently develop several GMP-grade nucleases (conventional: GMP-NUES19, salt active: GMP-NUES13). Both are ideal choices for removing nucleic acid contamination in your biopharmaceutical process flow.
 Wide range of salt concentration adaptation, maintains high enzyme activity under medium to high salt conditions
Wide range of salt concentration adaptation, maintains high enzyme activity under medium to high salt conditions High stability, retains enzyme activity at 37°C for 21 days without impairment
High stability, retains enzyme activity at 37°C for 21 days without impairment Downstream processes do not require dialysis or desalination, saving time
Downstream processes do not require dialysis or desalination, saving time Produced under GMP conditions, meeting the requirements from research to large-scale production
Produced under GMP conditions, meeting the requirements from research to large-scale production High enzyme activity
High enzyme activity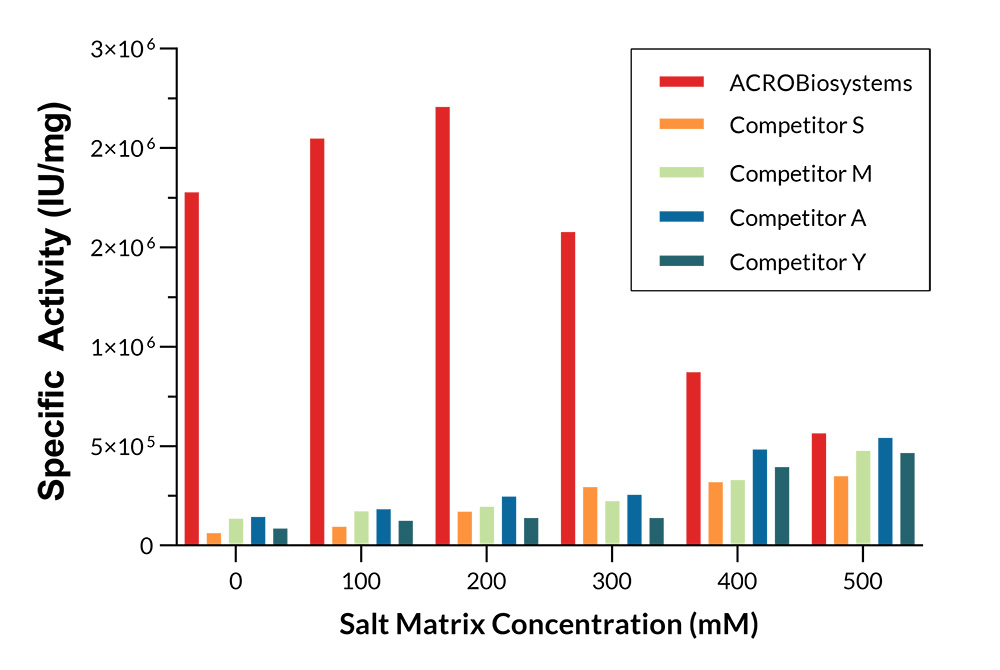
GMP Salt Active GENIUS ™ Nuclease (Cat. No. GMP-NUES13) can maintain high enzyme activity under 0-500mM NaCl conditions; The enzyme activity of GMP-NUES13 under 0-500mM conditions is significantly better than that of competitors.
 High nucleic acid removal effect
High nucleic acid removal effect 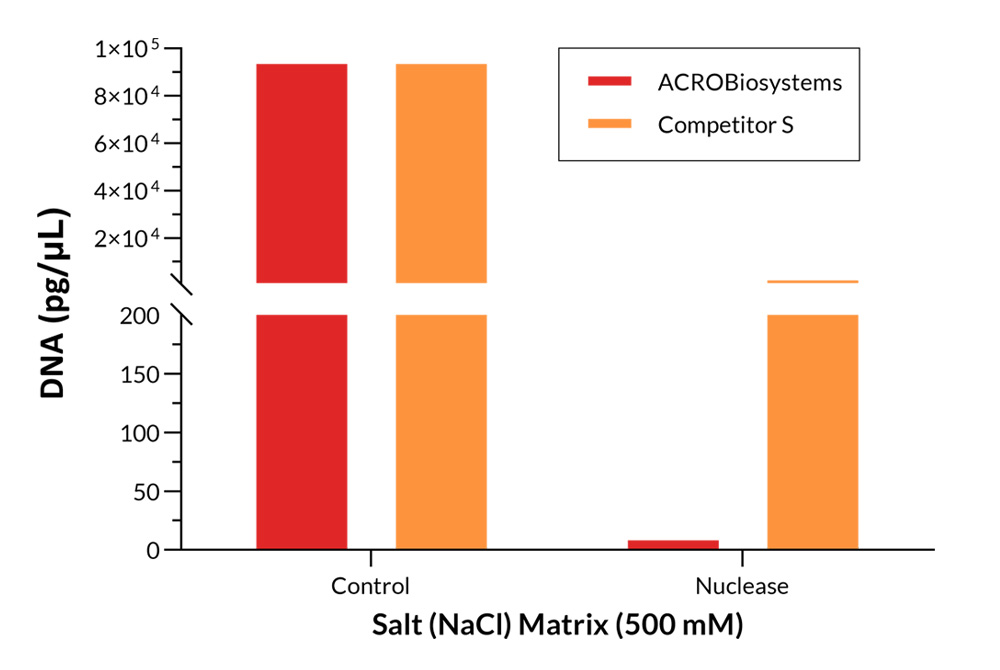
Using a cell homogenate containing 6.5E+06cell/mL HEK293, host cell DNA residue was detected by qPCR after being treated with 40U/mL salt active nuclease for 30 minutes. The results showed the DNA residues after treatment with GMP Salt Active GENIUS ™ (Cat. No. GMP-NUES13) is extremely low, and the nucleic acid clearance effect is better than that of competitors.
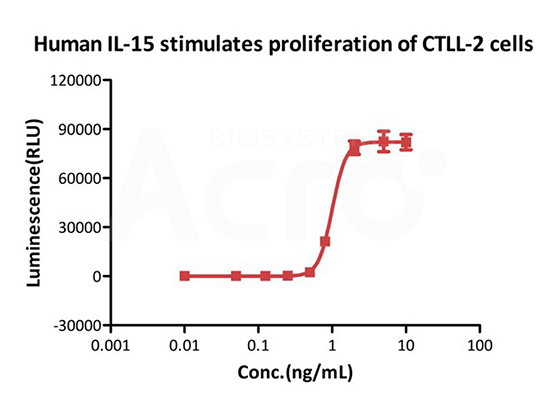
GMP Human IL-15 Protein (Cat. No. GMP-L15H13) stimulates proliferation of CTLL-2 cells. The specific activity of GMP Human IL-15 is > 8.00ⅹ10^6 IU/mg, which is calibrated against human IL-15 WHO International Standard (NIBSC code: 95/554) (QC tested).
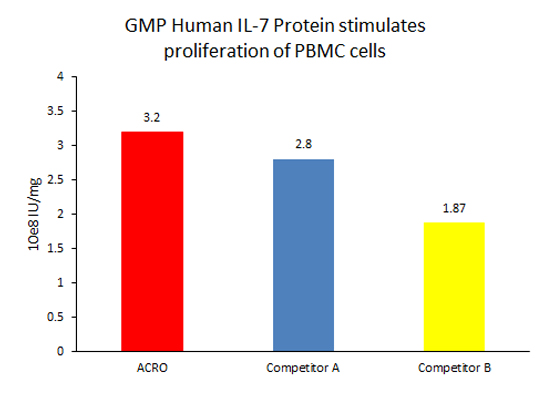
The activity of GMP Human IL-7 Protein (Cat. No. GMP-L07H24) was higher than other competing products.
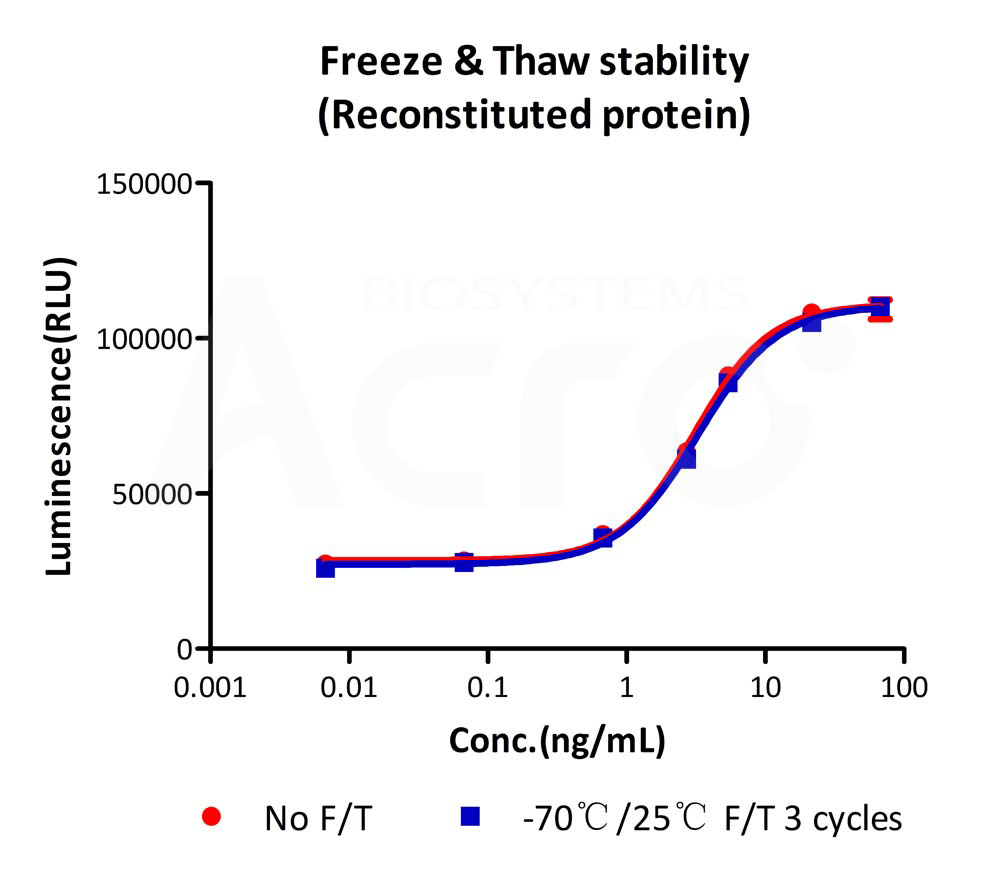
The Cell based assay shows that GMP Human IL-7 Protein (Cat. No. GMP-L07H24) is stable after freezing and thawing 3 times.
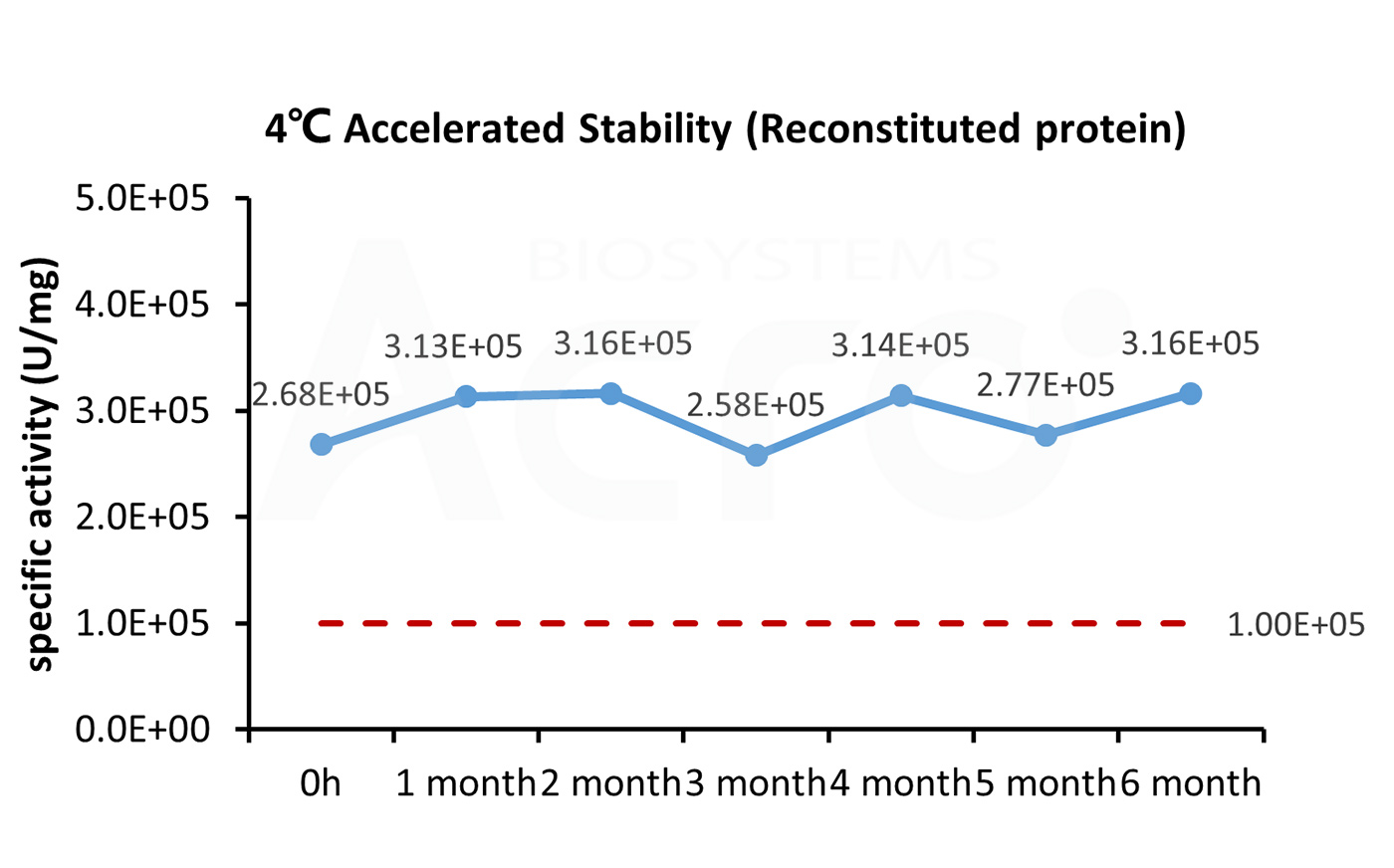
The Cell based assay shows that GMP Human IL-21 Protein (Cat. No. GMP-L21H25) is stable at 4°C for 6 months.
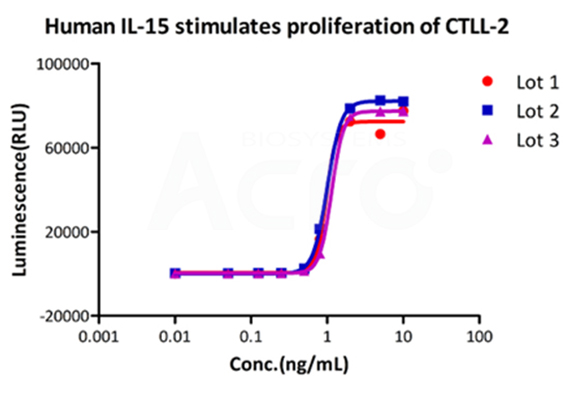
The cell activity of GMP Human IL-15 (Cat. No. GMP-L15H13) remains consistent across different batches and exhibits high batch to batch consistency.

This web search service is supported by Google Inc.
