 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Anti-Idiotypic Antibody Development Service Anti-idiotypic antibodies are antibodies that target the variable region of another antibody to produce specific binding. These antibodies are widely used in drug development: they can be used as an important reference standard for immunogenicity analysis or can be developed to specifically detect antibody drug levels for pharmacokinetic studies.
ACROBiosystems is committed to providing high quality key reagents and related services required in the development of targeted therapeutic drugs. We provide a series of anti-idiotypic antibodies with high affinity, high specificity, and high sensitivity. To meet the more unique needs of our customers, we also provide a one-stop service, starting from antigen preparation to monoclonal / polyclonal anti-idiotype antibody and pharmacokinetic / immunogenicity test kit development.

Hardware Strength
Through the international AAALAC certification, we can standardize the management and use of animals to ensure the quality of antibodies.

Efficient Project Management
Dedicated one-on-one service with our project team with fast response and follow-up.

High Quality Materials
Key reagents for PK/ADA analysis are provided for a variety of different types of therapeutic drugs including monoclonal antibodies, bispecific antibodies, ADCs, and CAR-T).

Multifunctional Service
Services starting from antigen preparation to monoclonal / polyclonal anti-idiotype antibody development and PK/immunogenicity testing kit development.

Compliant with Regulatory Guidelines
We ensure that our PK/ADA test kits are delivered with sensitivity that meets regulatory requirements.

We provide custom development of anti-idiotypic antibodies that are either antigen-neutralizing, non-neutralizing or drug target compound types. Depending on your application, these monoclonal anti-idiotypic antibodies can be used in the detection of free, bound, or total drug concentration in pharmacokinetic studies.

For immunogenicity studies, we provide high-affinity and specific polyclonal anti-idiotypic antibody development services. These highly sensitive and diverse polyclonal anti-idiotypic antibodies can be used as a positive reference in immunogenicity studies, simulating the production of ADA inside the human body.
In order to further support and facilitate your preclinical and clinical research of antibody drugs, we also offer our ELISA kit development services for immunogenicity (anti-drug antibody, ADA) or pharmacokinetic (PK) assays. Immunogenicity assays are used to quantify the level of immune response against the targeted therapeutic, while pharmacokinetic assays are used to elucidate the drug concentration and drug clearance within the body. Both assays are critical regulatory requirements for drug development, IND applications, clinical trials and post-marketing monitoring.
ACROBiosystems provides custom development services for both drug concentration and ADA detection kits for customers' diversified needs and applications while also providing key reagents for PK and immunogenicity analysis of biological drugs.
| Name | Period | Delivery | Price |
|---|---|---|---|
Anti-idiotypic rabbit polyclonal antibody preparation |
8-10 weeks |
|
One-on-one service of the project team Click here to contact us |
Anti-idiotypic mouse monoclonal antibody preparation |
4-5 months |
|
|
|
Development of a quantitative detection kit for blood drug concentration |
5-7 weeks |
|
| Molecule | Cat. No. | Antigen | Neutralizing Activity | Application |
|---|---|---|---|---|
| Adalimu*ab | ADB-Y19 | Anti-Adalimu*ab Antibodies (AY19) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y10 | Anti-Bevacizu*ab Antibodies (AY10) (MALS verified, recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y12 | Anti-Bevacizu*ab Antibodies (AY12) (recommended for neutralizing assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-Y9 | Anti-Bevacizu*ab Antibodies (AY9) (recommended for ADA assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Bevacizu*ab | BEB-BY13 | Biotinylated Anti-Bevacizu*ab Antibodies (AY13) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA;Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y27 | Anti-Cetuxi*ab Antibodies (AY27) (recommended for ADA assay) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y31 | Anti-Cetuxi*ab Antibodies (AY31) (Non-Neutralizing) | Non-Neutralizing Antibody | ADA assay; Indirect ELISA |
| Cetuxi*ab | CEB-Y28 | Anti-Cetuxi*ab Antibodies (AY28) | Neutralizing Antibody | ADA assay; Neutralizing assay; Indirect ELISA |
| Cetuxi*ab | CEB-BY31 | Biotinylated Anti-Cetuxi*ab Antibodies (AY31) (recommended for PK/PD) | Non-Neutralizing Antibody | PK bridging ELISA; Indirect ELISA |
| Rituxi*ab | RIB-Y36 | Anti-Rituxi*ab Antibodies (AY36) (recommended for ADA assay) | Neutralizing Antibody | ADA assay;Neutralizing assay; Indirect ELISA |
| Rituxi*ab | RIB-Y37 | Anti-Rituxi*ab Antibodies (AY37) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA;Neutralizing assay;Indirect ELISA |
| Rituxi*ab | RIB-FY35c | FITC-Labeled Anti-Rituxi*ab Antibodies, Mouse IgG1 | Neutralizing Antibody | ADA assay;Neutralizing assay; Indirect ELISA |
| Trastuzu*ab | TRB-Y5b | Anti-Trastuzu*ab Antibodies (AY5b) (recommended for PK/PD) | Non-Neutralizing Antibody | Neutralizing Antibody |
| Trastuzu*ab | TRB-Y1b | Anti-Trastuzu*ab Antibodies (AY1b) (recommended for PK/PD) | Neutralizing Antibody | PK bridging ELISA; Neutralizing assay; Indirect ELISA |

1. Antigen recheck (1 to 2 days)
SDS-PAGE purity recheck, UV concentration recheck
2. Antigen preparation (1 week, optional)
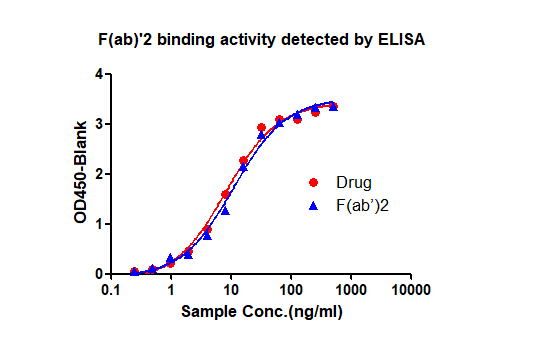
The customer provides full-length antibody drugs, we perform enzymatic digestion and purification to obtain F(ab) '2, and analyze the obtained F(ab)'2 for purity and activity, and provide a report on antigen preparation

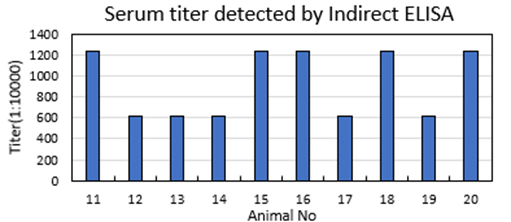
3. Immunization and titer detection (8 weeks)
Blood collection prior to immunization, 10 Balb/c mice immunization, titer detection, final blood collection, Immunization schedule and titer detection report provided; titer meets 1:72,9000

4. Cell fusion and screening (6-8 weeks)
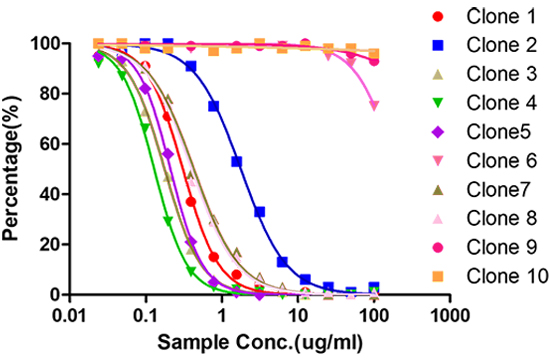
Spleen B cells from one or two mice with high titer are selected for electrofusion with SP2/0, positive clones screened by full-length antibody, isotype control antibody and human IgG are detected by indirect ELISA blocking ELISA is verifed cloning of competing and non-competing targets and performed 2-3 rounds of subcloning

5. Antibody production and pairing (2-3 weeks)
Hybridoma cell culture micro-purification and labeling, Sandwich paired validation, SDS-PAGE purity analysis, UV concentration determination, ELISA activity analysis and identification, PK assay development using sandwich method

6. Scale-up of antibody production and assay establishment (2-3 weeks)
Optimized paired antibodies are mass-produced, purified by SDS-PAGE analysis, UV concentration determination, ELISA activity analysis, PK Assay (standard curve, sensitivity, matrix interference validation, etc.) are established, and sensitivity meets the clinical PK assay requirements
7. Deliverables
- Serum before immunization, cellular supernatant
- Purified antibodies, cell lines
- Titer test report, fusion screening report, antibody identification report, COA, detailed project development report

1. Antigen recheck (1 to 2 days)
SDS-PAGE purity recheck, UV concentration recheck
2. Antigen check (1 to 2 days)
The customer provides full-length antibody drugs, we perform enzymatic digestion and purification to obtain F(ab) '2, and analyze the obtained F(ab)'2 for purity and activity, and provide a report on antigen preparation

3. Immunization and titer detection (8 weeks)
Blood collection before immunization, immunization of New Zealand white rabbits, titer detection, final blood collection, Immunization schedule and titer detection report provided; titer meets 1:72,9000
4. Antibody purification (2-3 weeks)
- Antigen affinity chromatography
- Total human IgG purification
- Purification of isotype IgG (optional)
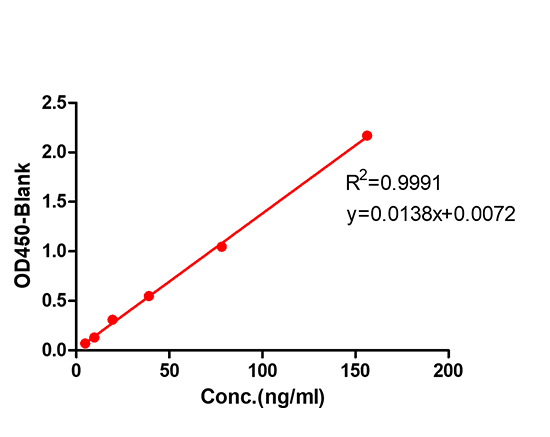

5. Antibody identification and Assay establishment (1 week)
- SDS-PAGE purity analysis, UV concentration determination
- Cross-reaction with isotype Human IgG is identified and analyzed by ELISA activity; cross-reactivity <2%
- ADA Assay establishment (sensitivity, MRD, etc.), sensitivity meets regulatory requirements.

6. Kit development
7. Deliverables
- Rabbit serum before immunization
- Purified antibodies, developed Kit
- Titer test report, antibody identification report, COA, detailed project development report
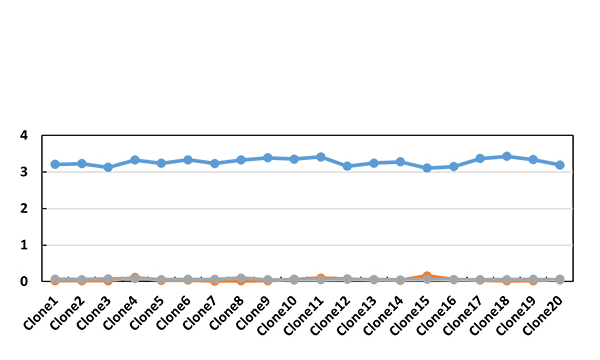
| Screened blocking clones against Arm 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone1 | Clone2 | Clone3 | Clone4 | Clone5 | Clone6 | Clone7 | Clone8 | Clone9 | Clone10 | A0 | |
| OD | 0.142 | 0.063 | 0.107 | 0.143 | 0.208 | 0.264 | 0.23 | 0.148 | 0.149 | 0.22 | 1.601 |
| blocking rate | 91.13% | 96.06% | 93.32% | 91.07% | 87.01% | 83.51% | 85.63% | 90.76% | 90.69% | 86.26% | / |
| Clone11 | Clone12 | Clone13 | Clone14 | Clone15 | Clone16 | Clone17 | Clone18 | Clone19 | Clone20 | A0 | |
| OD | 0.295 | 0.228 | 0.119 | 0.098 | 0.078 | 0.229 | 0.516 | 0.709 | 0.923 | 0.965 | 1.601 |
| blocking rate | 81.57% | 85.76% | 92.57% | 93.88% | 95.13% | 85.7% | 67.77% | 55.72% | 42.35% | 39.73% | / |
| Screened blocking clones against Arm 2 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Clone1 | Clone2 | Clone3 | Clone4 | Clone5 | Clone6 | Clone7 | Clone8 | Clone9 | Clone10 | A0 | |
| OD | 0.109 | 0.232 | 0.178 | 0.158 | 0.146 | 0.276 | 0.245 | 0.106 | 0.165 | 0.198 | 1.546 |
| blocking rate | 92.95% | 84.99% | 88.49% | 89.78% | 90.56% | 82.15% | 84.15% | 93.14% | 89.33% | 87.19% | / |
| Clone11 | Clone12 | Clone13 | Clone14 | Clone15 | Clone16 | Clone17 | A0 | ||||
| OD | 0.48 | 0.495 | 0.387 | 0.658 | 0.064 | 0.651 | 1.062 | 1.546 | |||
| blocking rate | 68.95% | 67.98% | 74.97% | 57.44% | 95.86% | 57.89% | 31.31% | / | |||
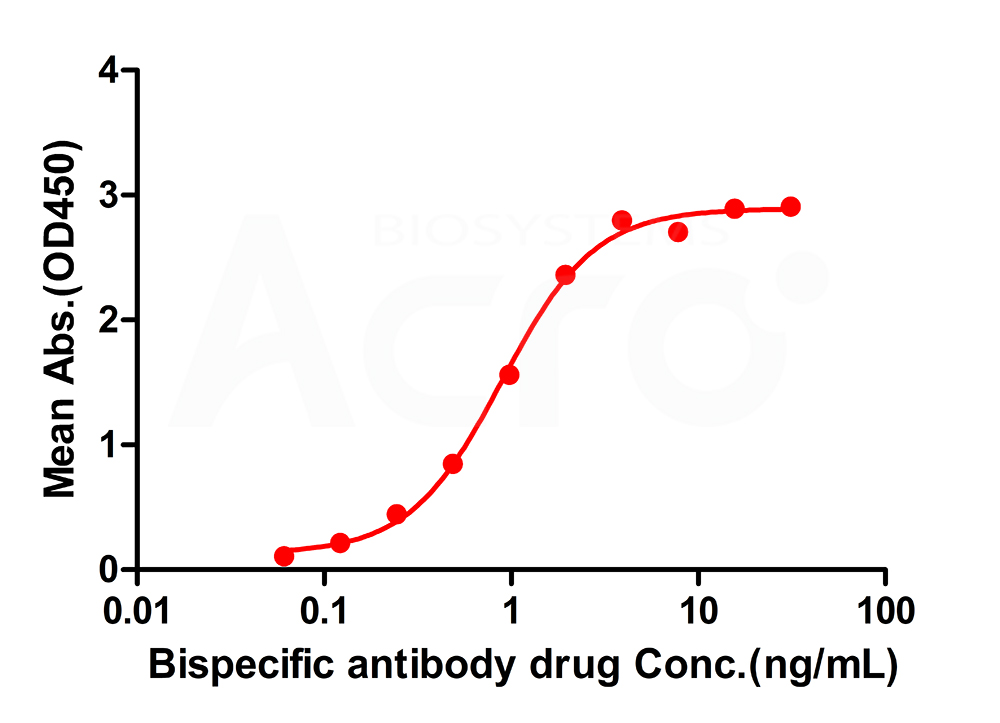
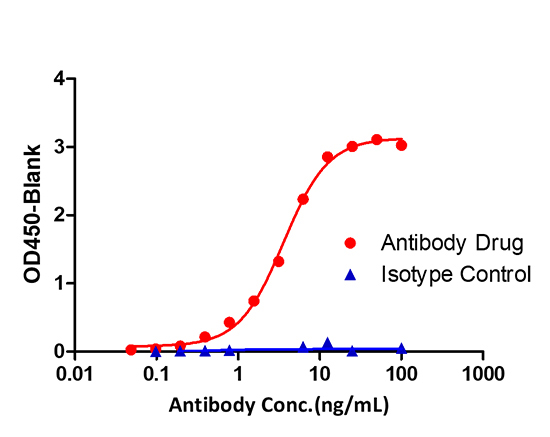
Immobilized anti-arm1 antibody can bind Bispecific antibody drugs, and then add Biotin- anti-arm2 antibody. Detection was performed using HRP-conjugated streptavidin with sensitivity of 0.24 ng/mL (Intact Assay).
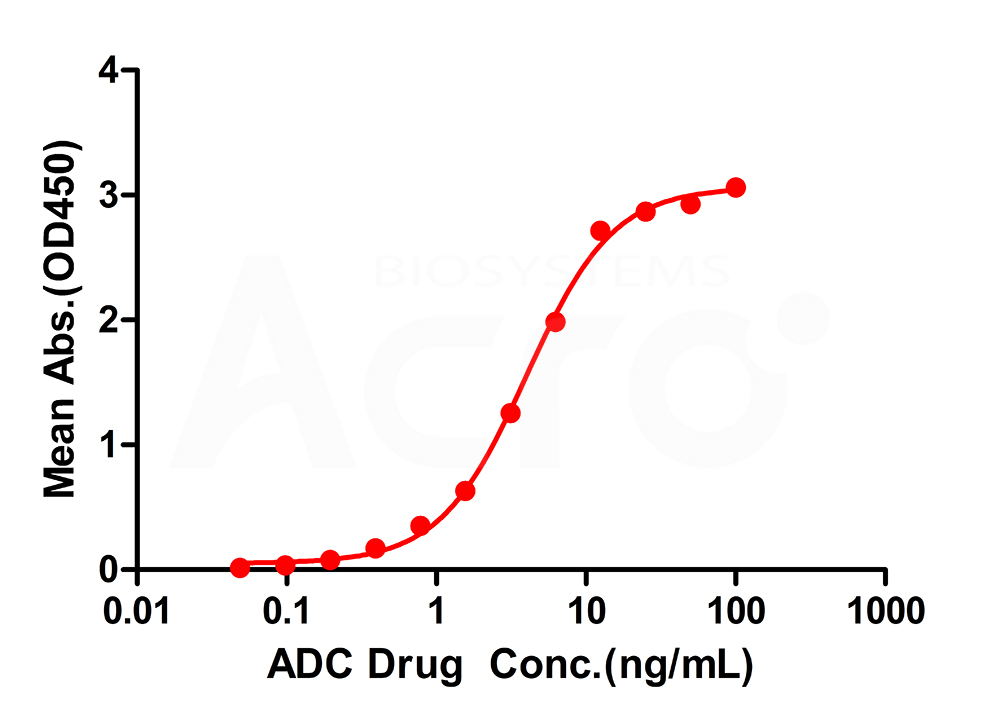
Immobilized Anti- drug Antibody, Mouse IgG1 can bind ADC antibody drugs, and then add Biotin- Mouse Anti-MMAE Antibody, Mouse IgG1 (MALS verified). Detection was performed using HRP-conjugated streptavidin with sensitivity of 0.69 ng/mL (Intact Assay).
| Testing method | Coated | Sample | Testing |
| Antigen capture ELISA | CD20 | Antibodies to be tested | Goat anti-human IgG |
| Anti-idiotypic capture ELISA | Anti-Ritux*mab Antibodies | Antibodies to be tested | Goat anti-human IgG |
| Bridging ELISA by anti-idiotypic antibodies | Anti-Ritux*mab Antibodies | Antibodies to be tested | Biotinylated Anti-Ritux*mab Antibodies |
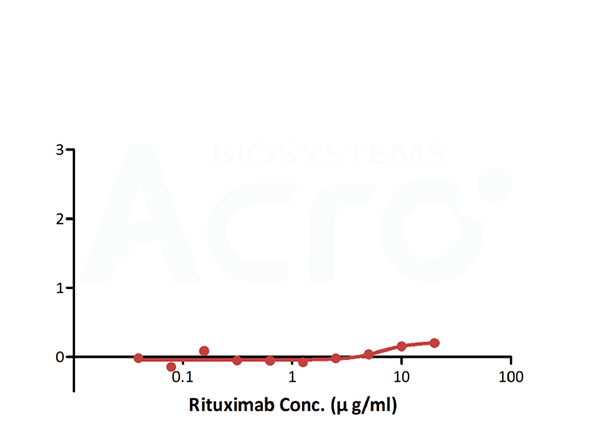
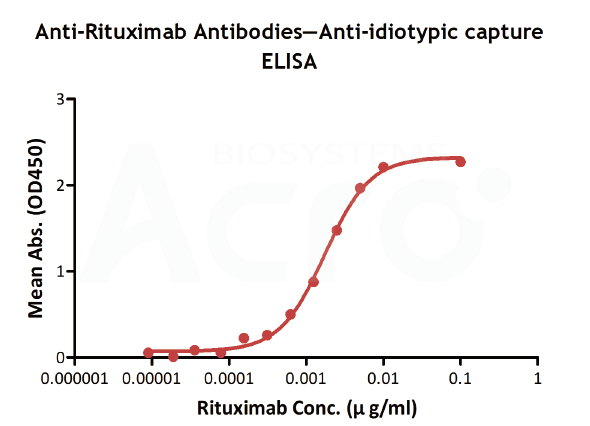
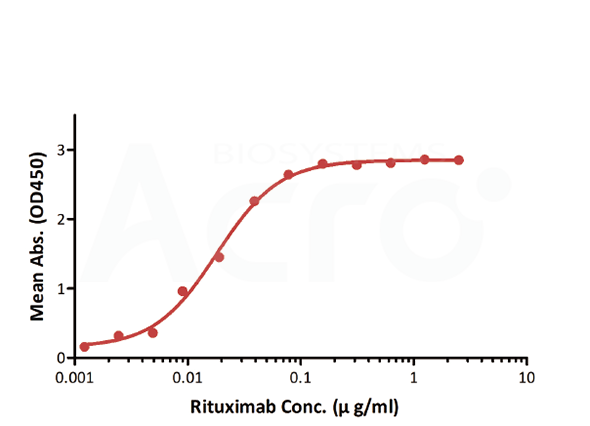
| Testing method | Linear range (µg/mL) |
Sensitivity (µg/mL) |
Advantage | Disadvantage |
| Antigen-capture ELISA | — | — | Simple method and good versatility | High background, no activity |
| Anti-idiotypic capture ELISA | 0.156-10 | 0.156 | Solve the difficulty in obtaining CD20, simple method | High background, only suitable for Rituxa biosimilar |
| Bridging ELISA by anti-idiotypic antibodies | 0.012-0.78 | 0.012 | Solve the difficulty in obtaining CD20, good sensitivity and low background | Only applicable to Rituxan biosimilar |
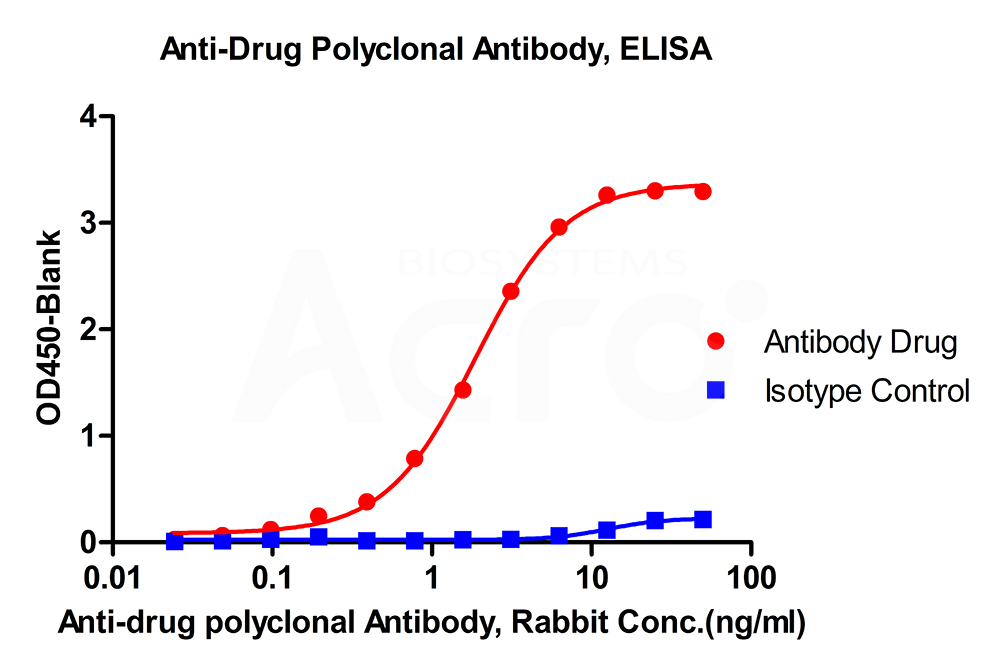
New Zealand white rabbits were immunized with full-length monoclonal antibodies. The antiserum was affinity purified for polyclonal antibodies specific to the drug. Cross reactivity to subtype control was less than 2%.
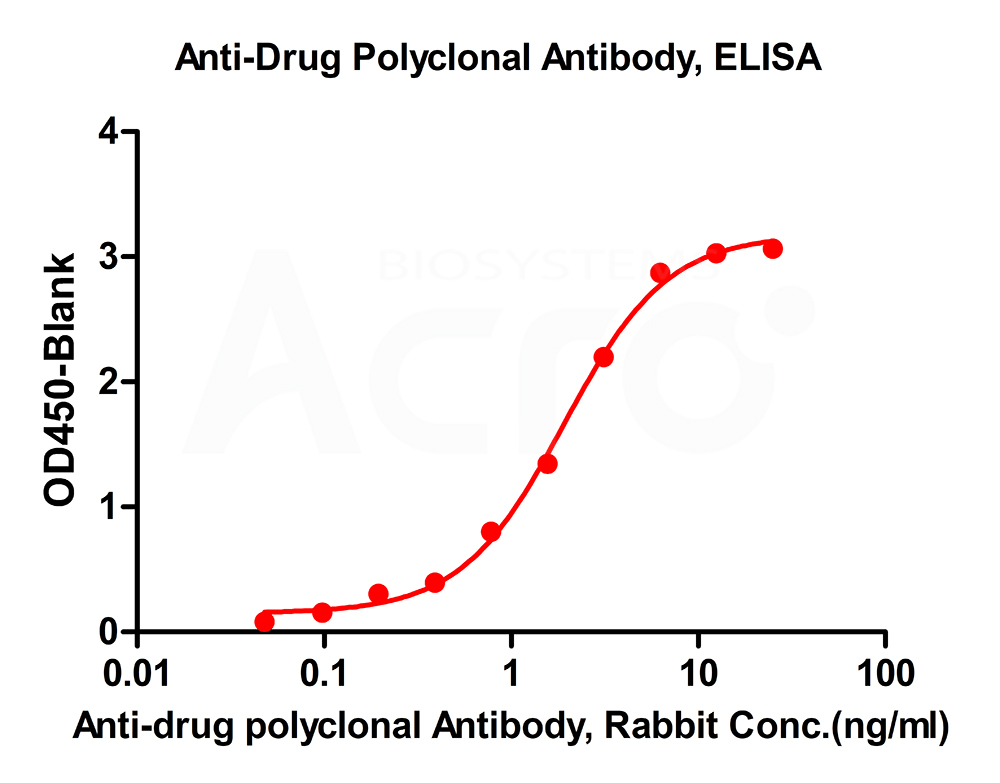
New Zealand white rabbits were immunized with polypeptide-KLH conjugates. Antiserum was purified by affinity chromatography until antibody titer was more than 1:712,000.
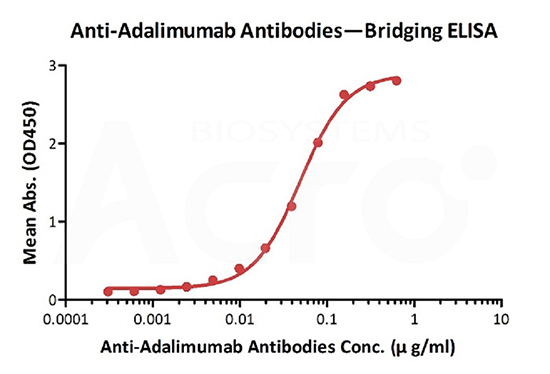
Anti-Adalimumab Antibodies bridging ELISA for Anti-Drug Antibody (ADA) assay development. Immobilized adalimumab at 1 µg/ml, add increasing concentrations of Anti-Adalimumab Antibodies (Cat. No. ADB-Y19, 10% human serum) and then add biotinylated adalimumab at 5 µg/ml. Detection was performed using HRP-conjugated streptavidin with a sensitivity of 0.6 ng/mL.
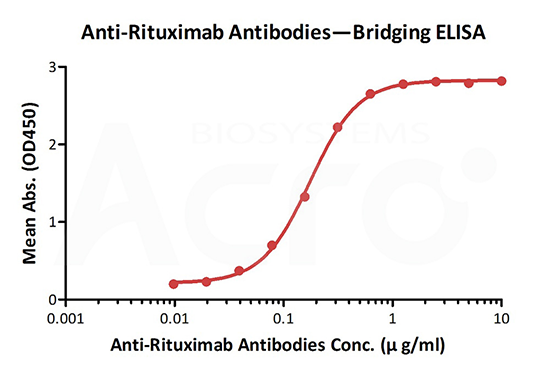
Anti-Rituximab Antibodies bridging ELISA for Anti-Drug Antibody (ADA) assay development. Immobilized rituximab at 1 µg/ml, added increasing concentrations of Anti-Adalimumab Antibodies (Cat. No. RIB-Y36, 10% human serum) and then added biotinylated rituximab at 2 µg/ml. Detection was performed using HRP-conjugated streptavidin with a sensitivity of 9.7 ng/mL.
ACROBiosystems scientist will respond within 24 hours of submission.
Call us at: +1 800-810-0816 or email at services@acrobiosystems.com for consultation.
This web search service is supported by Google Inc.
