 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> IL-2 and IL-2 Receptor Proteins 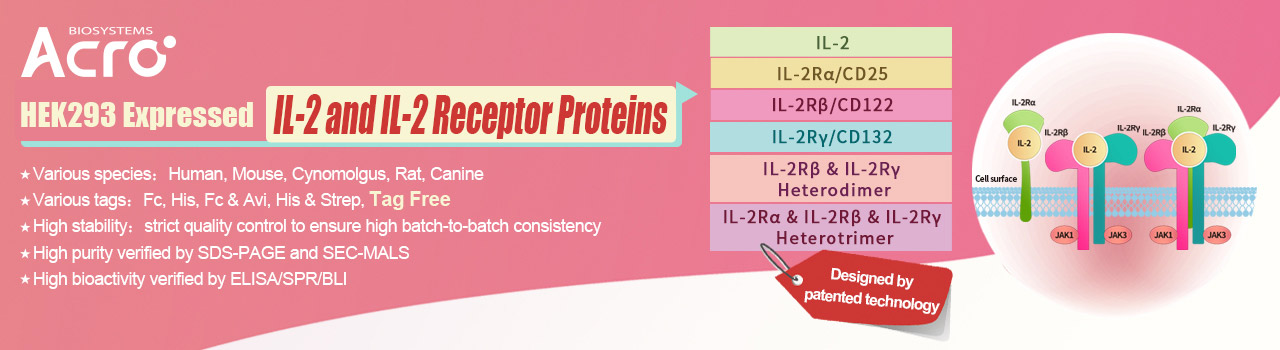
Interleukin-2 (IL-2) is a pluripotent cytokine which plays a crucial role in the immune system response. And the IL-2 receptor (IL-2R) is a heterotrimeric protein expressed on the surface of certain immune cells, such as lymphocytes, that binds and responds to IL-2.
IL-2R consists of three subunits, namely IL-2Rα (CD25), IL-2Rβ (CD122), and common γc (CD132). The three receptor chains are expressed separately and differently on various cell types and can assemble in different combinations and orders to generate low, intermediate, and high affinity IL-2 receptors.
Many biopharma and biotech companies have carried out different IL-2 modification and design, including PEG modification, fusion Fc, IL-2 mutant design, bispecific antibody design, and combined immunization checkpoint antibody drug therapy and other strategies.
Therefore, a series of structurally stable and high-affinity IL-2 receptor heterodimers and heterotrimers are of great significance for in vitro studies of the interaction between IL-2 and IL-2 receptors, and also for the antibody immunization and screening.
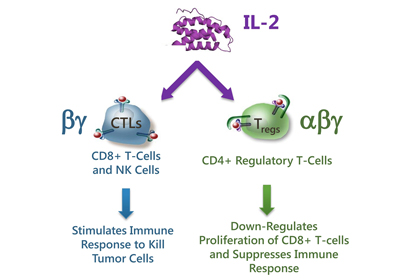
Fig.1 Native IL-2 has pleiotropic effects on the immune response[1]
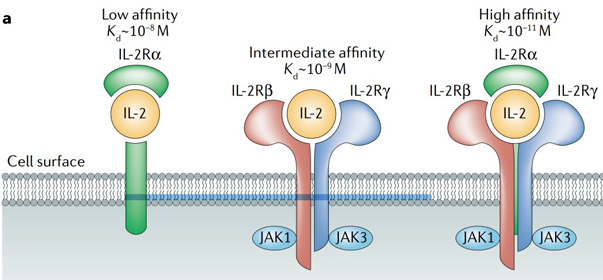
Fig 2. Schematic of IL-2 binding to the low-affinity, intermediate-affinity and high-affinity IL-2 receptors. The high-affinity receptor comprises IL-2 receptor α-chain (IL-2Rα), IL-2Rβ and IL-2Rγ.[2]
ACROBiosystems has developed a series of IL-2 related proteins: IL-2, IL-2Rα (CD25), IL-2Rβ (CD122), IL-2Rγ (CD132), IL-2 R βγ heterodimer, IL-2Rαβγ heterotrimer with high purity, high stability and high bioactivity, which can be used to research the interaction between IL-2 and IL-2 receptors, immunization, antibody screening, etc., to facilitate the development of IL-2 related drugs. In addition, we can also provide GMP-grade IL-2 used for scale-up culturing of various cell types such as T/NK cells and iPSCs.
| Molecule | Cat. No. | Host | Product Description | Structure |
|---|
| Molecule | Cat. No. | Host | Product Description | Structure |
|---|
| Molecule | Cat. No. | Host | Product Description | Structure |
|---|
| Molecule | Cat. No. | Host | Product Description | Structure |
|---|
| Molecule | Cat. No. | Host | Product Description | Structure |
|---|
| Molecule | Cat. No. | Host | Product Description | Structure |
|---|
Table 1 Affinity of IL-2 binding to IL-2R (SPR) |
||||
IL-2 R |
||||
Affinity to Human IL-2 |
40.6 pM |
0.279 nM |
29.9 nM |
377 nM |
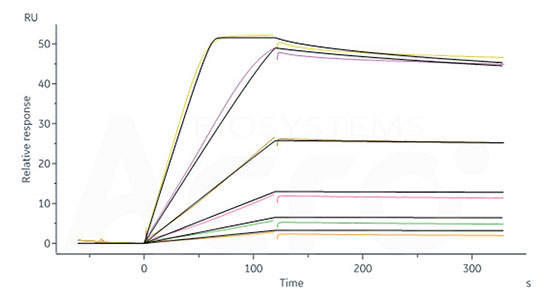
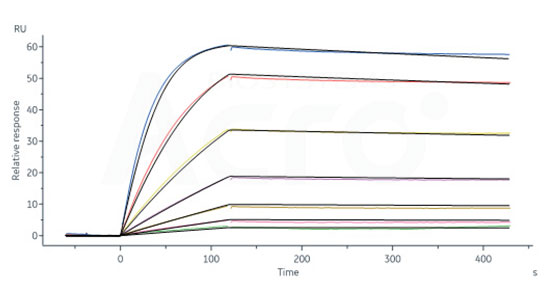
Human IL-2RB&IL-2RA&IL-2RG, Fc Tag&Fc Tag (Cat. No. ILG-H5257) captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind Human IL-2, Tag Free with an affinity constant of 40.6 pM as determined in a SPR assay (Biacore 8K) (Routinely tested).
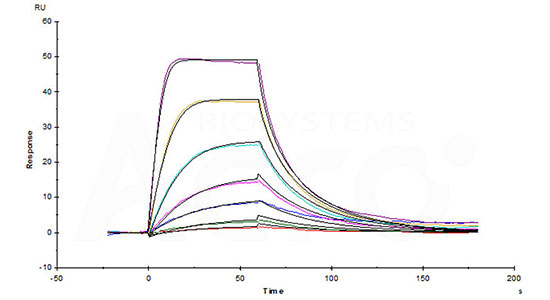
Human IL-2RB&IL-2RG Heterodimer Protein, Fc Tag&Fc Tag (Cat. No. ILG-H5254) captured on CM5 chip via Anti-human IgG Fc antibodies surface can bind Human IL-2, Tag Free with an affinity constant of 0.279 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
Human IL-2 R alpha, His Tag (Cat. No. ILA-H52H9) captured on CM5 chip via anti-His antibody, can bind Human IL-2, Tag Free with an affinity constant of 29.9 nM as determined in a SPR assay (Biacore T200) (Routinely tested).
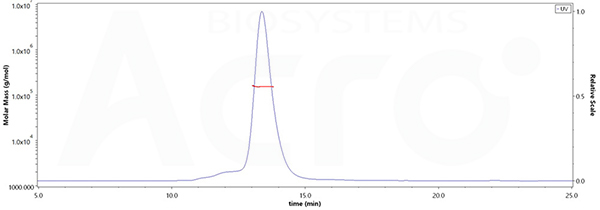
The purity of Human IL-2RB&IL-2RG Heterodimer Protein, Fc Tag&Fc Tag (MALS verified)(Cat. No. ILG-H5254) is more than 90% and the molecular weight of this protein is around 145-165 kDa verified by SEC-MALS.
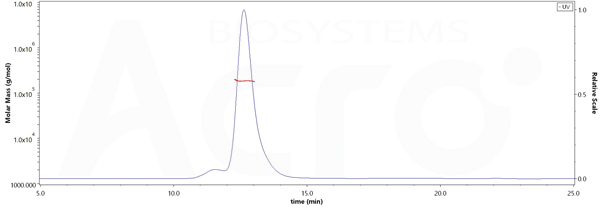
The purity of Human IL-2RB&IL-2RA&IL-2RG, Fc Tag&Fc Tag(Cat. No. ILG-H5257) is more than 90% and the molecular weight of this protein is around 175-190 kDa verified by SEC-MALS.
Reference:
1. NKTR-2017JPMorganSlides
2. Spolski R, et al. Nat Rev Immunol. 2018. PMID: 30089912 Review.
3. Xinquan Wang et al.,2005, Science. 310(5751):1159-63.
4. Balasubramanian, S. Int Immunol. 1995 Nov;7(11):1839-49.
This web search service is supported by Google Inc.
