 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Recombinant Antibody Services Antibodies are widely used in biomedical research, not only as therapeutic drugs, but also in immunodiagnostics and various biologics-based analytical research. Recombinant antibodies, unlike the traditional hybridoma antibodies, have become popularized due to its unique advantages in customization and large-scale antibody production. Through genetic engineering, antibodies are developed directly from an antibody gene sequence to eliminate traditional immunization and antibody screening steps, resulting in high-throughput, large-scale antibody production.
As a supplier of key reagents in the global biomedical field, ACROBiosystems develops and produces high-quality recombinant antibody reagents to meet the growing demand for custom antibody reagents in early drug discovery and preclinical research. Relying upon ACROBiosystem’s independently-optimized mammalian cell expression platform and production experience in antibody reagent products, we provide our customers with recombinant antibody expression and production services based on HEK293 and CHO cells. We provide a comprehensive recombinant antibody development service including gene construction, expression, purification, and quality inspection. This means starting from the sequence information of antibodies (or VH/VL) and delivering your custom recombinant antibody with high purity and low endotoxin level.
High-throughput Recombinant Antibody Expression Service
• CHO/HEK293-based Cell Expression Platform
• 2-3 weeks to delivery of purified antibodies.
• Application-based customizable service including production method, concentration, etc.
Antibody Class Switching Service
• One-stop service from gene construction to expression from antibody (or VH/VL) sequence information.
• Antibody class switching services are available. (e.g. switching from hybridoma mouse antibody to human antibody IgG1/2/3/4).
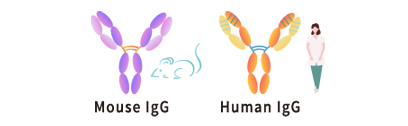
Large-scale Recombinant Antibody Production Service
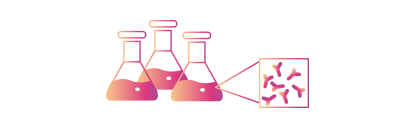
• Large-scale Production services supporting gram to kilogram of recombinant antibodies
• Low Endotoxin levels: <1EU/mg.
• 50L-200L Bioreactors
• Customizable production plan to support the customer needs.
 |
||||||
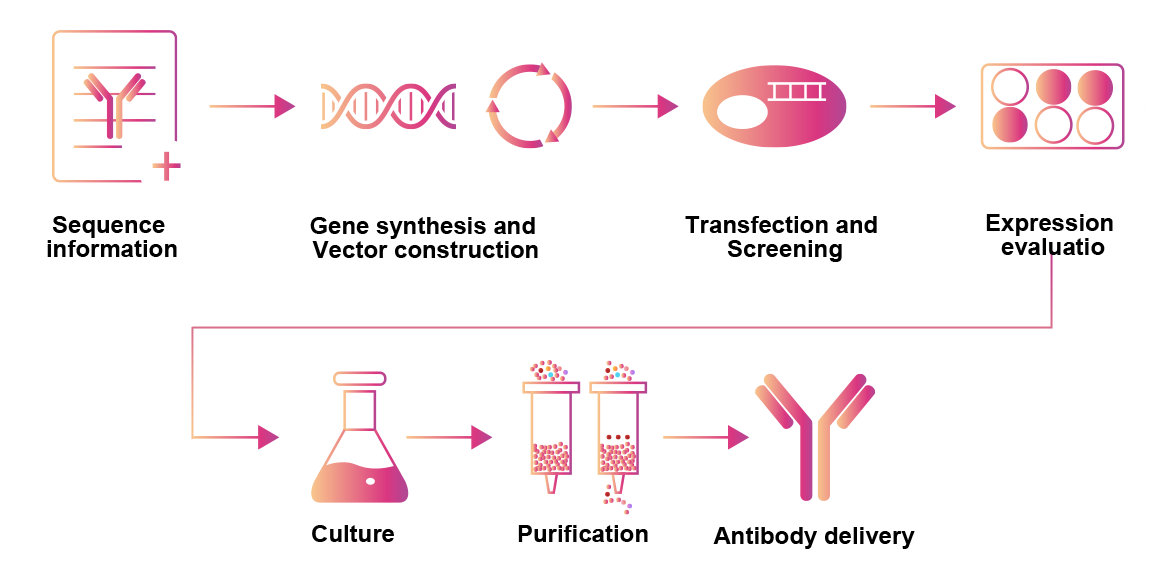
Sequence information |
Gene synthesis and Vector construction |
Transfection and Screening |
Expression evaluation |
Culture |
Purification |
Antibody delivery |
Gene synthesis
(optional)
~1 week
• Codon optimization
• Gene synthesis
Vector construction
~1 week
• Vector construction
• Plasmid extraction
Antibody expression
and purification
1 week
• Cell transient transfection
• Antibody expression and purification
Quality control
and delivery
3 week
• Purified recombinant antibody
• Quality inspection report, CoA
Codon optimization
Gene synthesis
Vector construction
Plasmid extraction
Cell transient transfection
Antibody expression and purification
Purified recombinant antibody
Quality inspection report, CoA
 |
|||
Optimized mammalian cell expression platform: |
Multiplexed Antibody Development: |
High quality standard Purity: |
High-throughput: |
 |
||
Flexible Deliverables: |
Large-scale Production Capacity: |
Powerful Analytical and Detection Technology Platforms: |
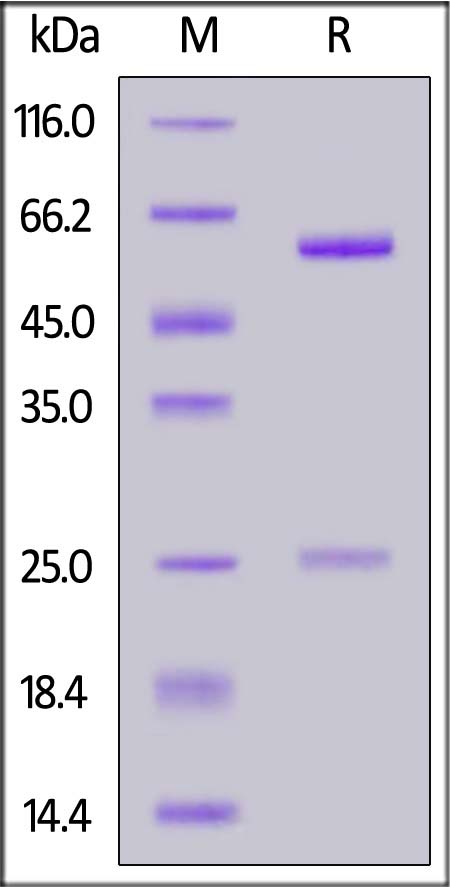
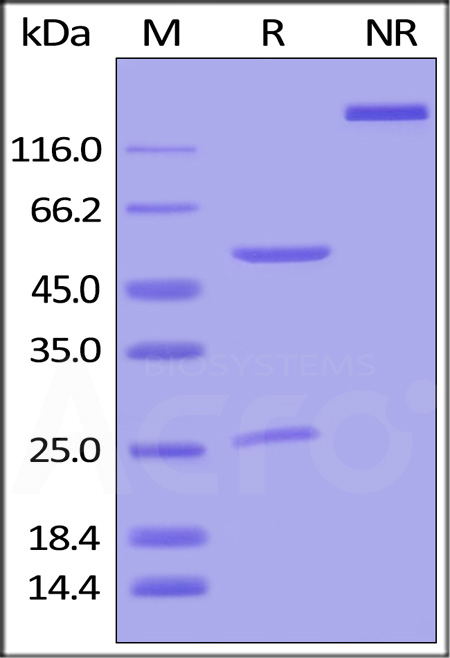
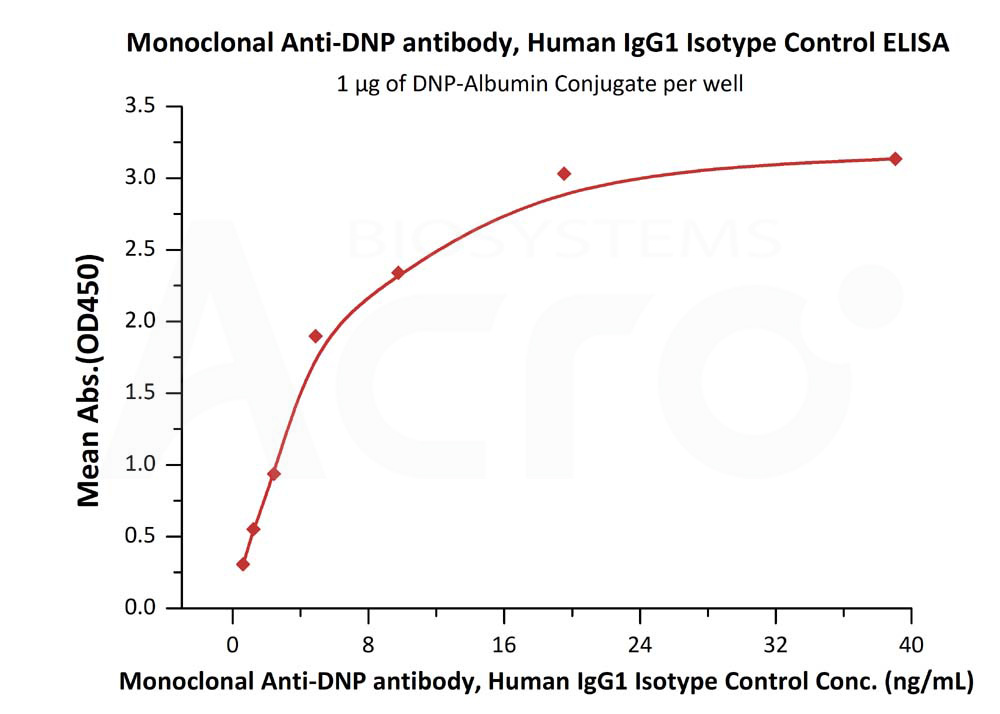
Immobilized DNP-Albumin Conjugate at 10 μg/mL (100 μL/well) can bind Human IgG1 Kappa Isotype Control (mAb) (Cat. No. DNP-M2) with a linear range of 1-10 ng/mL (QC tested).
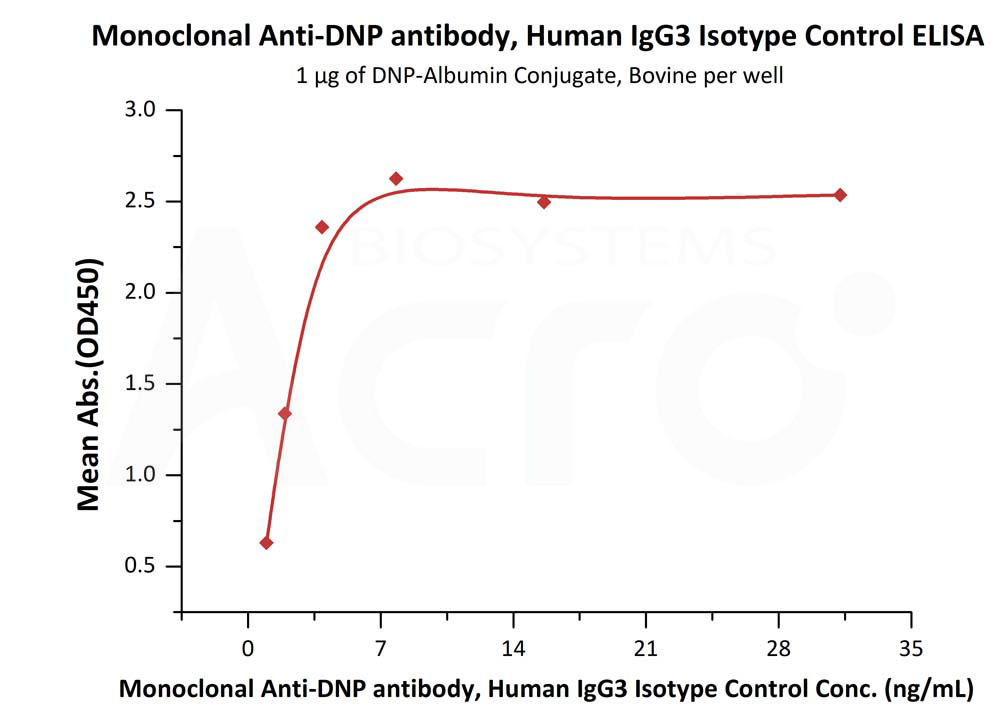
Immobilized DNP-Albumin Conjugate, Bovine at 10 μg/mL (100 μL/well) can bind Human IgG3 Kappa Isotype Control (mAb) (Cat. No. DNP-M915) with a linear range of 0.1-4 ng/mL (QC tested).
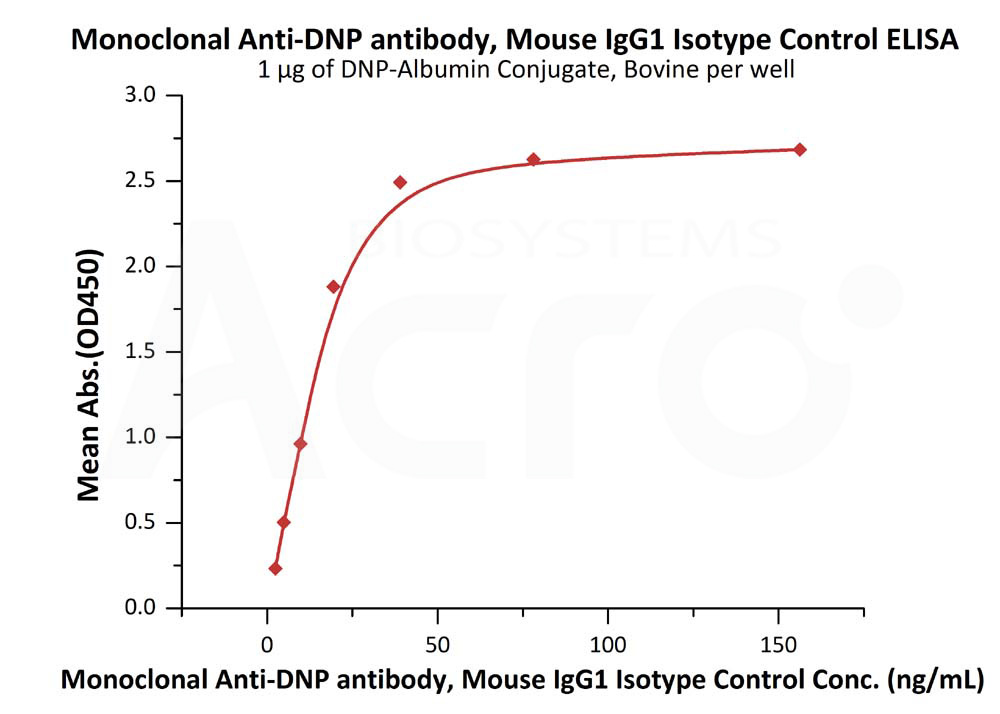
Immobilized DNP-Albumin Conjugate, Bovine at 10 μg/mL (100 μL/well) can bind Mouse IgG1 Kappa Isotype Control (mAb) (Cat. No. DNP-M1) with a linear range of 2-20 ng/mL (QC tested).
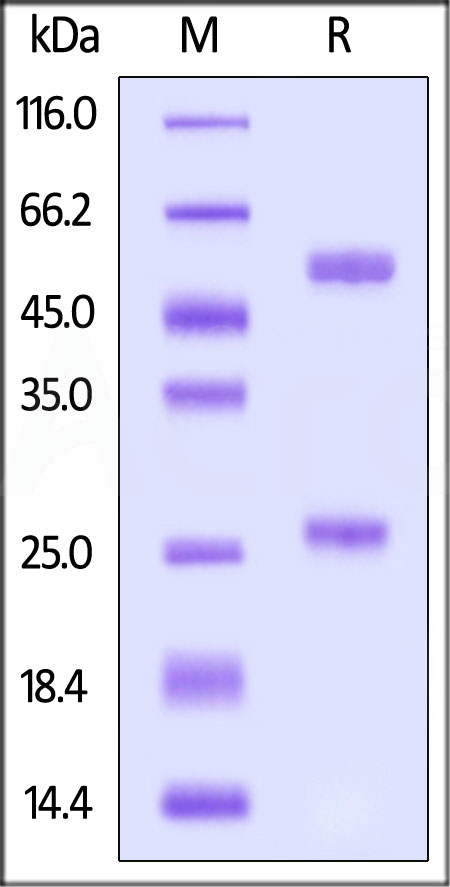
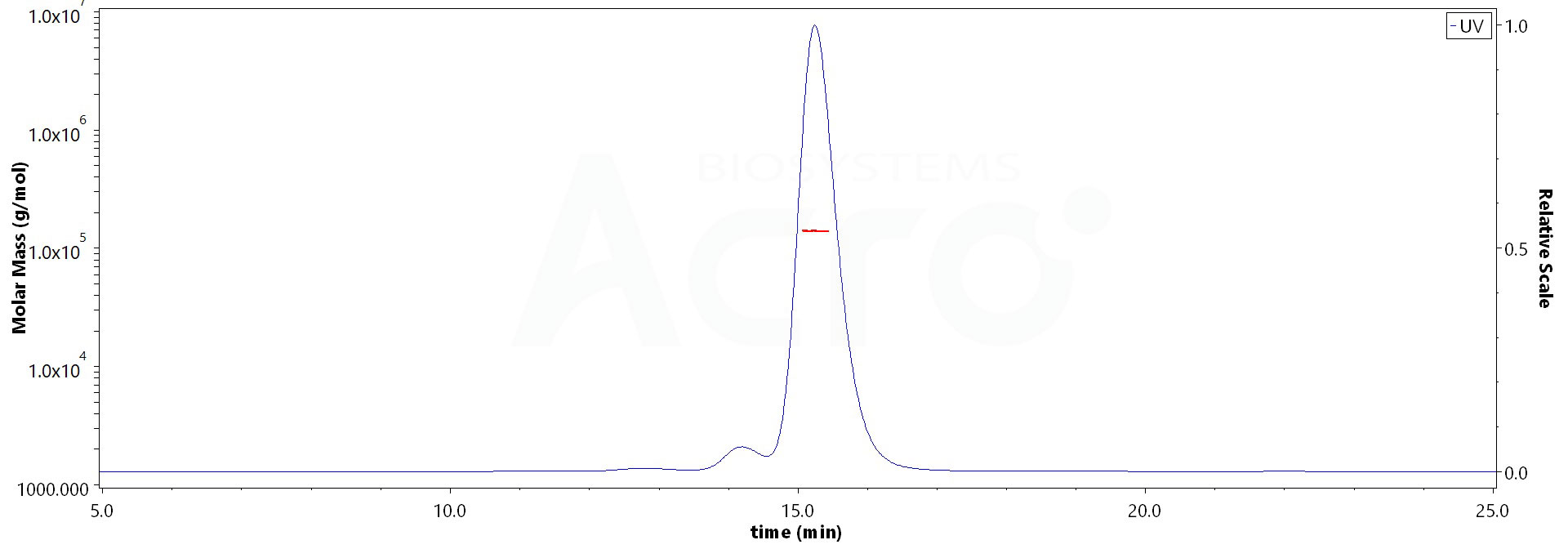
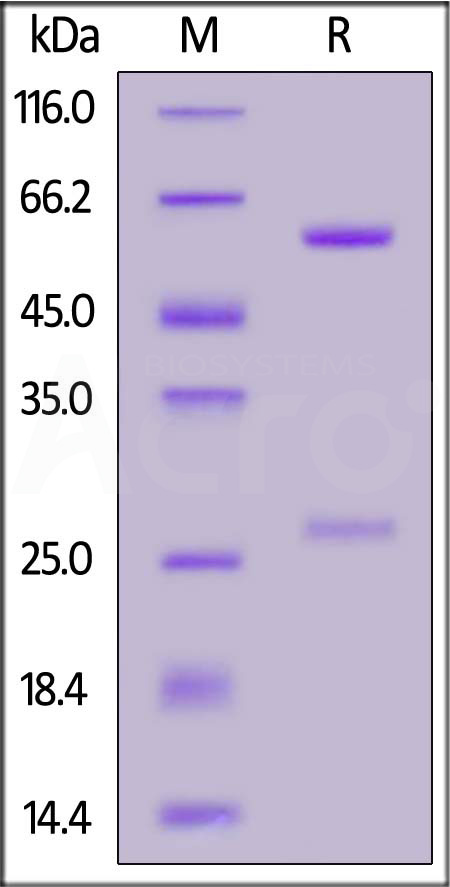
| Source | Isotype | Purity | Endotoxin |
| CHO cells | Human IgG1/kappa | >95% as determined by SDS-PAGE |
<0.1EU/mg |
ACROBiosystems scientist will respond within 24 hours of submission.
Call us at: +1 800-810-0816 or email at order@acrobiosystems.com for consultation.
This web search service is supported by Google Inc.
