 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Antibody drug application > SPR & BLI: Analysis Platforms for Protein Binding Activity Surface Plasmon Resonance (SPR) works via an incident light striking the interface of two media with different refractive indexes at a critical angle which may cause metal free-electron resonance. Due to this resonance, the electrons absorb the light energy, which greatly weakens the reflection light at a certain angle. The angle of incidence that makes the reflected light disappear completely within a certain angle is called the SPR angle. The range of SPR varies with the refractive index of the surface, which is in turn proportional to the mass of biomolecules bound to the metal surface. Therefore, the dynamic changes of SPR angle in biological reactions can be obtained by specific signals of the interaction between biomolecules.
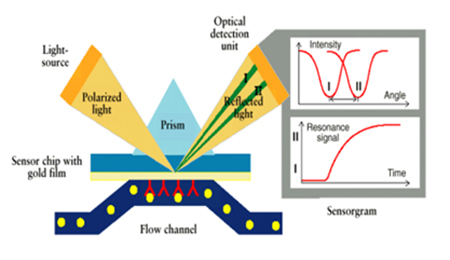

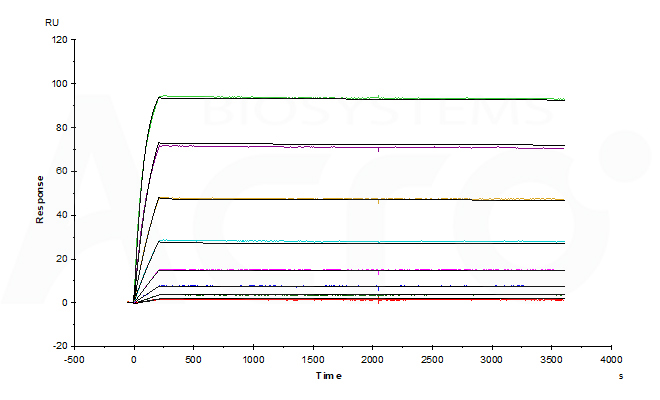
Fig 3-A Anti-Adalim*mab Antibodies (mouse IgG1, Cat. No. ADB-Y19) captured on CM5 chip via anti-mouse antibodies surface can bind human adalim*mab with an affinity constant of 1.36 pM.
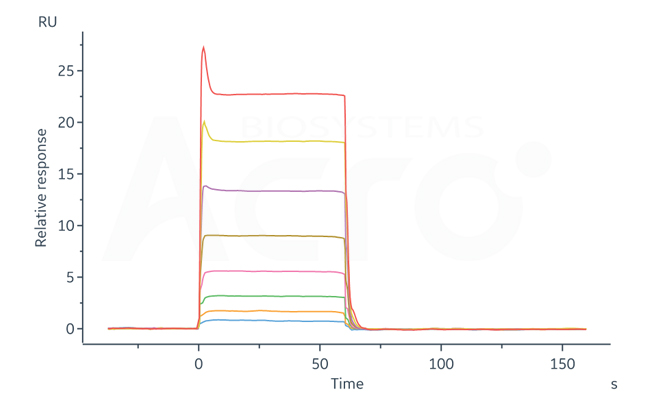
Fig 3-B Human CD32a Protein, Strep Tag (Cat. No. CDA-H5284) immobilized on CM5 Chip can bind Rituximab with an affinity constant of 1.47 μM as determined in a SPR assay.
Biofilm Interference Technology (BLI) can achieve real-time monitoring of intermolecular interactions with biosensors. After light passes through the biofilm layer of the sensor, transmission and reflection will occur; the frequency of reflected light is affected by the thickness of the biofilm. Some frequencies of reflected light interfere constructively with the incident light, while others interfere destructively. The spectrometer detects these interference light waves and forms an interference spectrum, which is displayed as the interference spectrum's relative displacement intensity (nm). Therefore, once the number of molecules bound to the sensor surface increases or decreases, the spectrometer will detect the displacement of the interference spectrum in real-time. This displacement directly reflects the thickness of the biosensor surface.
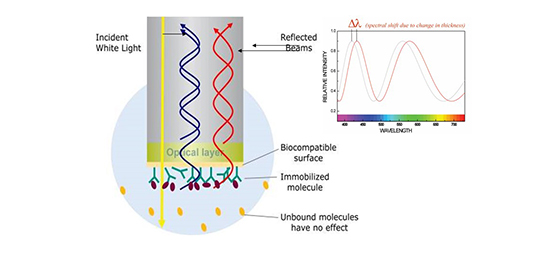

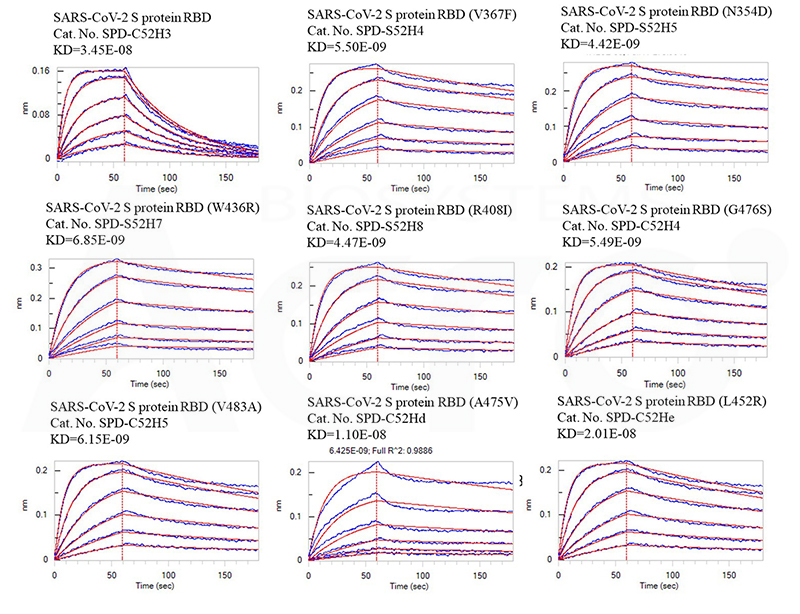
Fig 6 Affinity test between RBD mutants, variants, and Human ACE2 by BLI assay
ACROBiosystems is committed to providing high-quality recombinant proteins with highly comprehensive production and quality control platforms. Our team is committed to providing the most suitable solutions and resources to fit our customer's application, experimental design, and support protein verification. One way to do this is by providing detailed protocols at no extra cost to show the steps and details of detection and ultimately save time, materials, and efforts on our customer's experimental design and planning.
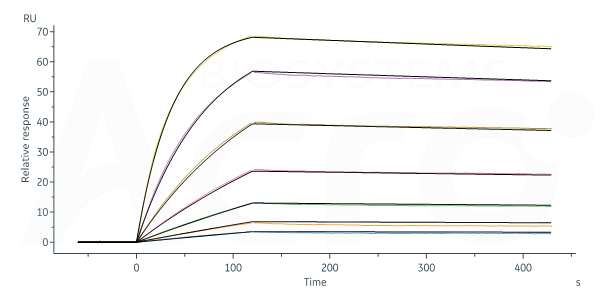
Fig. 8-A Herc*ptin captured on Protein A Chip can bind Human CD64, His Tag (Cat. No. FCA-H52H1) with an affinity constant of 0.108 nM as determined in SPR assay (Biacore 8K) (Routinely tested).
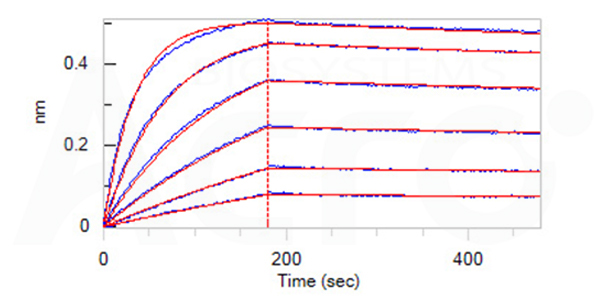
Fig. 8-B Loaded Herc*ptin on Protein A Biosensor, can bind Human CD64, His Tag (Cat. No. FCA-H52H1) with an affinity constant of 0.297 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
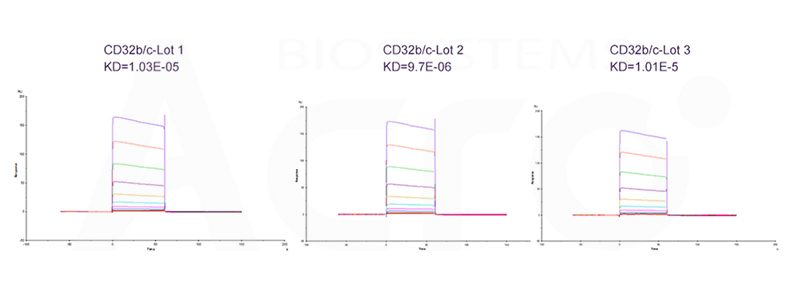
Figure 9. SPR verification, fixed to CM5 chip Human Fc gamma RIIB / CD32b Protein (Cat. number CDB-H5228) It can bind Ritux*mab with an affinity constant of 10 μM (Biacore T200). Protocol
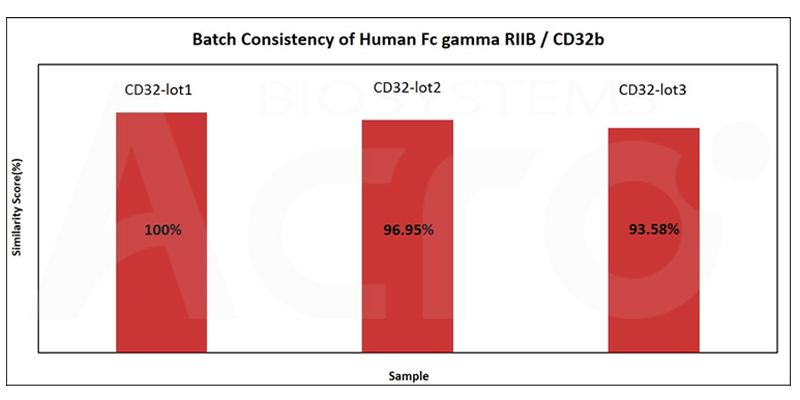
Figure 10. Using the analysis of similarity scoring software, different batches of Human Fc gamma RIIB / CD32b (Cat. No. CDB-H5228). The similarity is more than 90%
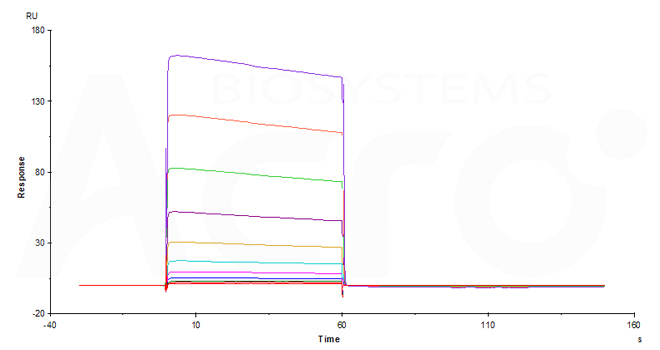
Fig. 11-A Immobilized Human CD32b/c, His Tag (Cat. No. CDB-H5228) on CM5 Chip via anti-His antibody can bind Ritux*mab with an affinity constant of 10.1 μM as determined in an SPR assay (Biacore T200) (QC tested).
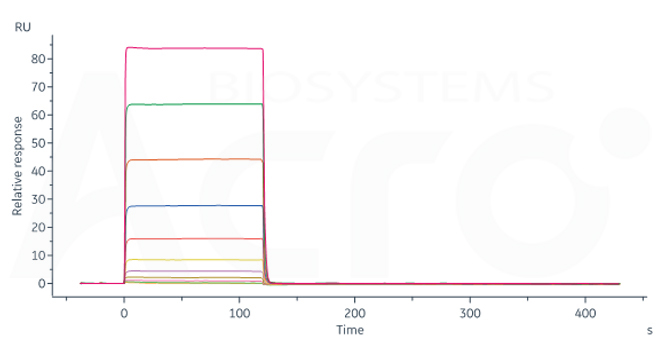
Fig. 11-B Ritux*mab immobilized on CM5 Chip can bind Human CD32b/c, His Tag (Cat. No. CDB-H5228) with an affinity constant of 4.09 μM as determined in SPR assay (Biacore 8K) (Routinely tested).
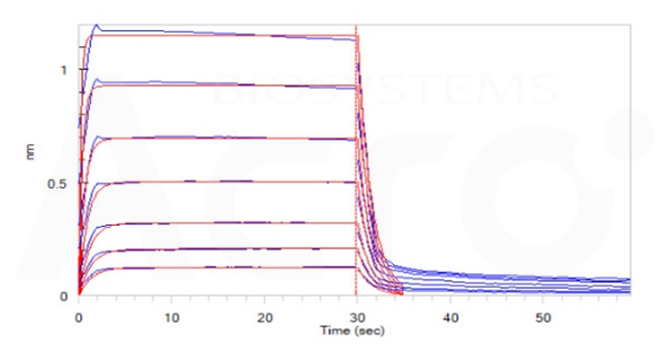
Fig. 11-C Loaded Human CD32b/c, His Tag (Cat. No. CDB-H5228) on HIS1K Biosensor, can bind Ritux*mab with an affinity constant of 4.30 μM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
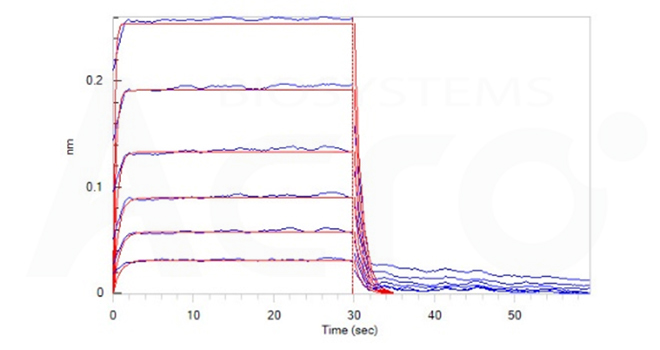
Fig. 11-D Loaded Ritux*mab on FAB2G Biosensor, can bind Human CD32b/c, His Tag (Cat. No. CDB-H5228) with an affinity constant of 5.4 μM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
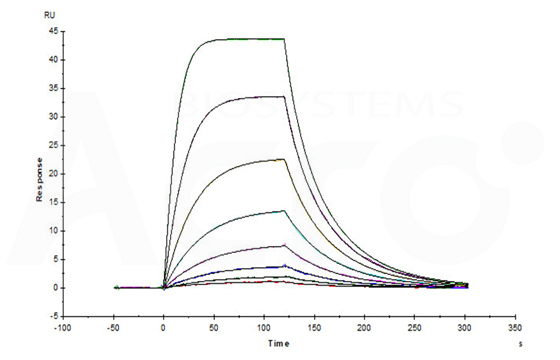
Fig 12-A Anti-Human BCMA MAb (human IgG1) captured on CM5 chip via Anti-Human IgG Fc antibodies surface, can bind Human BCMA, His Tag (Cat. No. BCA-H522y) with an affinity constant of 13.0 nM as determined in an SPR assay (Biacore T200) (Routinely tested).
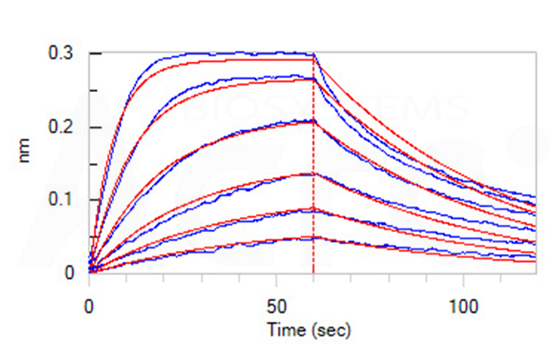
Fig 12-B Loaded Anti-Human BCMA MAb (human IgG1) on Protein A Biosensor, can bind Human BCMA, His Tag (Cat. No. BCA-H522y) with an affinity constant of 29.1 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
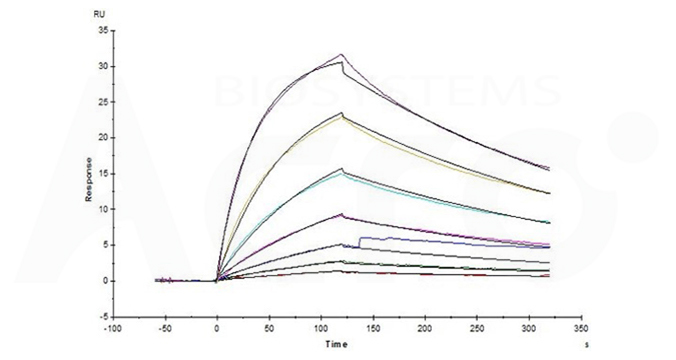
Fig.13 Bispecific T-cell Engager (CD3 X BCMA) immobilized on CM5 Chip can bind Human CD3E&CD3D Heterodimer Protein, His Tag&Tag Free (Cat. No. CDD-H52W1) with an affinity constant of 31.8 nM as determined in an SPR assay (Biacore T200).
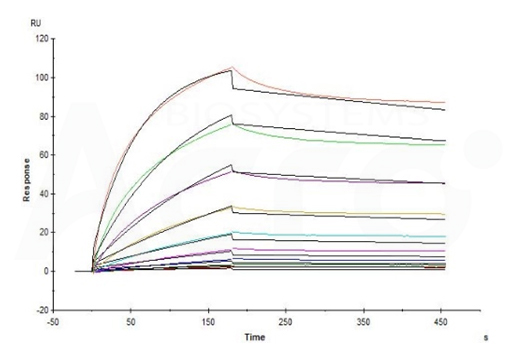
Fig 14-A Ritux*mab captured on CM5 chip via anti-human IgG Fc antibody can bind Cynomolgus CD20 Full Length, His Tag (Cat. No. CD0-C52H8) with an affinity constant of 5.48 nM as determined in an SPR assay (in the presence of DDM and CHS) (Biacore T200) (Routinely tested).
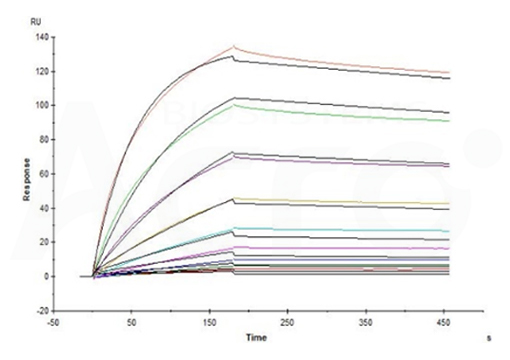
Fig 14-B Ofatumumab captured on CM5 chip via anti-human IgG Fc antibody can bind Cynomolgus CD20 Full Length, His Tag (Cat. No. CD0-C52H8) with an affinity constant of 3.51 nM as determined in an SPR assay (in the presence of DDM and CHS) (Biacore T200) (Routinely tested).
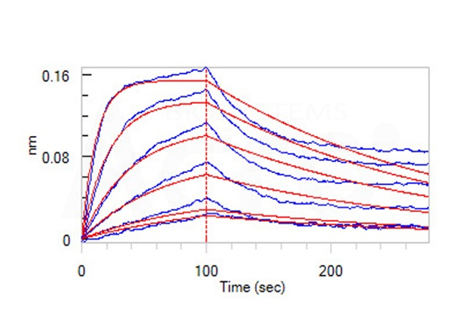
Fig 14-C Loaded Anti-Human CD47 MAb (Human IgG4) on AHC Biosensor, can bind Human CD47, His Tag (Cat. No. CD7-H5227) with an affinity constant of 6.18 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
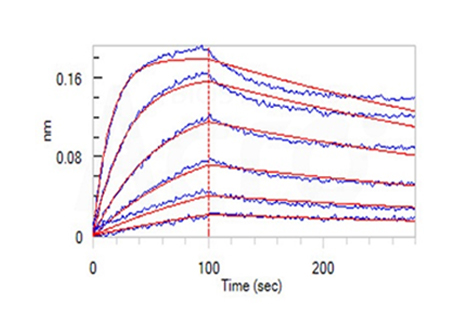
Fig 14-D Loaded Anti-Human CD47 MAb (Human IgG4) on AHC Biosensor, can bind Cynomolgus / Rhesus macaque CD47, His Tag (Cat. No. CD7-C52H1) with an affinity constant of 2.93 nM as determined in BLI assay (ForteBio Octet Red96e) (Routinely tested).
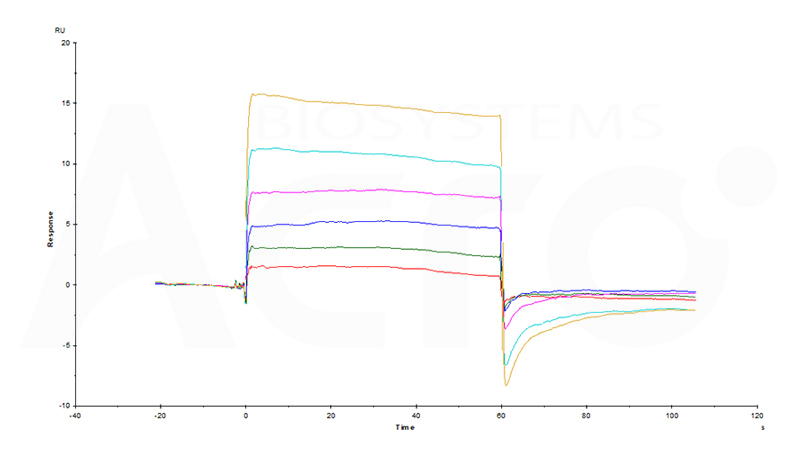
Fig 15 Human HSA Protein, His Tag (Cat. No. HSA-H5220) immobilized on CM5 Chip can bind Naproxen Sodium with an affinity constant of 41 μM as determined in an SPR assay (Biacore T200).
>>> Click to download more references about SPR/BLI affinity test cases
This web search service is supported by Google Inc.
