 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Core reagents for infectious disease research  RSV |  VZV |  RABV |  Influenza |  MPXV |  HIV |
 HSV |  EBV |  NiV |  HeV |  EBOV |  ZIKV |
 DENV |  SFTSV |  Coronavirus |  Rotavirus |  Coxsackievirus |  HCMV |
 MuV |  hMPV |  JEV |
>>> SARS-CoV-2 related products:Core reagents for COVID-19 vaccine R&D , Antigens & antibodies for diagnostic kits development , High-throughput solutions for vaccine R&D based on ELISA
>>> Hot Products: Hemagglutinin (HA), Neuraminidase (NA)
>>> Hot Products:Glycoprotein, Anti-RABV glycoprotein antibody
>>> Hot Products: Glycoprotein
>>> Hot Products: gp41, gp120, Capsid protein p24, HLA-A*0201 | B2M & HIV Gag (SLYNTVATL)
>>> Hot Products: Glycoprotein E
>>> Hot Products: NS1
>>> Hot Products: Glycoprotein G, Fusion glycoprotein F0 (Pre-fusion, Post-fusion)
>>> Hot Products: Glycoprotein, Fusion glycoprotein
>>> Hot Products:Gn protein
>>> Hot Products: gH&gL , Glycoprotein C (HSV-2), Glycoprotein D (HSV-1), Glycoprotein D (HSV-2), Glycoprotein E (HSV-2), post-gB
>>> Hot Products: gp350, Glycoprotein H & Glycoprotein L (EBV), Glycoprotein B (EBV)
>>> Hot Products: Pre-Fusion glycoprotein, Glycoprotein (NiV, HeV), ephrinB2
>>> Hot Products: Envelope protein, NS1
>>> Hot Products: VP4
>>> Hot Products: VP0
>>> Hot Products: Glycoprotein B / gB, gH&gL&gO:
>>> Hot Products: HN protein, Fusion glycoprotein F0, Mumps virus HN
>>> Hot Products: Post-Fusion glycoprotein F0
>>> Hot Products: Envelope protein E (JEV)
![]() HEK293 expression: the protein structure is closer to the natural conformation;
HEK293 expression: the protein structure is closer to the natural conformation;
![]() High purity verified by SDS-PAGE and MALS;
High purity verified by SDS-PAGE and MALS;
![]() High Bioactivity verified by antigen-antibody or antigen-receptor binding experiments;
High Bioactivity verified by antigen-antibody or antigen-receptor binding experiments;
![]() High immunogenicity, which can induce higher antibody titers;
High immunogenicity, which can induce higher antibody titers;
![]() It can be used for drug screening and vaccine development.
It can be used for drug screening and vaccine development.
RSV
influenza
VZV
MPXV
HIV
RABV
HSV
NiV
HeV
ZIKV
DENV
SFTSV
EBV
EBOV
Rotavirus
Coxsackievirus
LCMV
Vaccinia Virus
HCMV
hMPV
JEV
HPV
MuV
HTNV
TBEV
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
| product type | molecule | Cat. No. | Product Description |
|---|
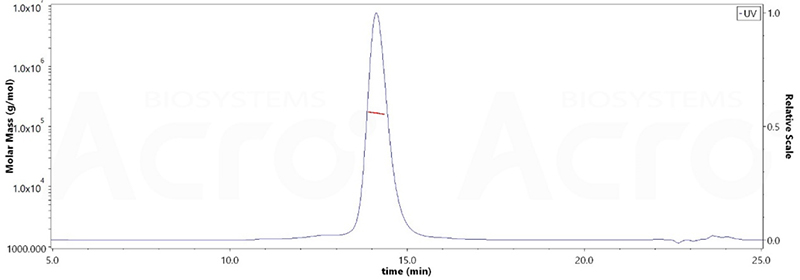
The purity of HRSV (A) Fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H6) is more than 95% verified by SDS-PAGE and 90% and verified by SEC-MALS. The molecular weight of this protein is around 148-182kDa.
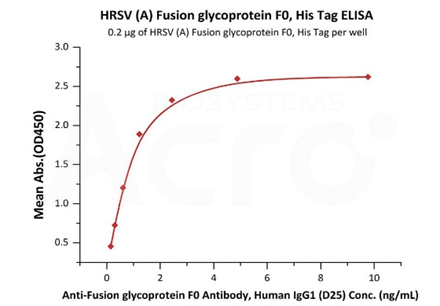
Immobilized HRSV (A) Fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H7) at 2 μg/mL (100 μL/well) can bind Anti-Fusion glycoprotein F0 Antibody, Human IgG1 (D25) with a linear range of 0.2-1 ng/mL (QC tested).
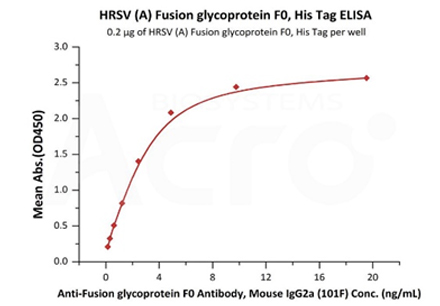
Immobilized HRSV (A) Fusion glycoprotein F0, His Tag (Cat. No. RSF-V52H7) at 2 μg/mL (100 μL/well) can bind Anti-Fusion glycoprotein F0 Antibody, Mouse IgG2a (101F) with a linear range of 0.2-5 ng/mL (Routinely tested).
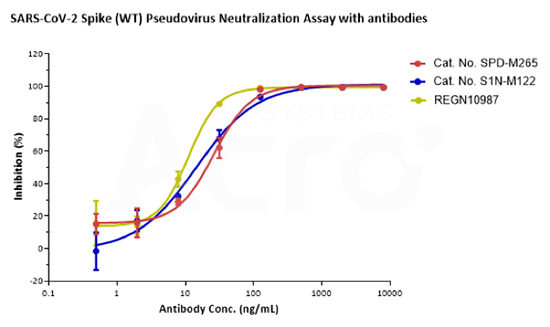
Loaded Monoclonal Anti-SARS-CoV-2 Spike RBD Antibody, Mouse IgG1 (Cat. No. SPD-M305) on AMC Biosensor, can bind SARS-CoV-2 Spike RBD, His Tag (B.1.1.529/Omicron) (Cat. No. SPD-C522e) with an affinity constant of 9.07 nM as determined in BLI assay (ForteBio Octet Red96e).
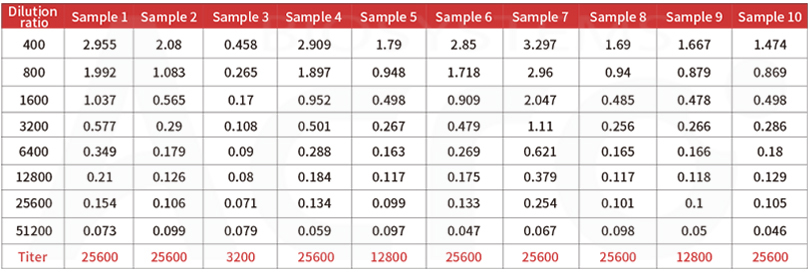
Post-vaccination serum samples are tested with Anti-SARS-CoV-2 Antibody IgG Titer Serologic Assay Kit (Spike RBD) (Cat. No.RAS-T024), which accurately and precisely measure antibody titer in serum (Accuracy≤±15%; Intra-assay precision<10%; Inter-assay precision <15%).
This web search service is supported by Google Inc.
