COVID-19 pandemic continues to impact individuals, families, and communities around the world. Early testing of the disease will lead to quick identification of cases, quick treatment for those people and immediate isolation to prevent spread. Diagnosis of COVID19 is also important in the bigger public health picture on mitigation efforts, helping investigators characterize the prevalence, spread, and contagiousness of the disease.
We currently have two main types of tests for COVID-19, which are the molecular test and the serological test. The molecular test, also known as the Nucleic Acid Amplification Tests (NAAT), detects the genetic material from SARS-CoV-2, which causes COVID-19. This method is recommended by the WHO and FDA as the gold standard for COVID-19 diagnosis.
However, the molecular test has its limitations. Detecting SARS-CoV-2 from pharyngeal swabs requires high-quality specimens that contain a sufficient amount of intact viral RNA. While on the other hand, the virus loads vary considerably in the respiratory tract, which has not only led to high false-negative rates, with probable cases remaining negative after multiple swabs but is further exposing healthcare workers to a risk of infection.
Unlike the molecular test, the serological test detects the antibodies in the blood sample, usually obtained through a finger prick, giving better patient compliance. Besides, the serological test can identify those that were infected and have recovered, which gives a broader picture of COVID-19 infection spread. Typically, the serological test is faster than the molecular test and does not require special equipment to process the results. Due to the importance and the convenience of the serological test, many countries around the world are planning to employ this method as an additional diagnostic tool. Germany, for example, is conducting nationwide testing for COVID-19 antibodies, becoming the first European country to do so.
The serological test can employ different formats, including colloidal immuno-chromatographic strip, enzyme-linked immunosorbent assay, and chemical immunoluminescence method, while the principles behind each format are pretty similar. Take the chemical immunoluminescence method as an example. The antigen immobilized beads can capture the specific antibodies from the patient sample (i.e. blood sera). After adding a secondary antibody reporter to the system, the virus-specific immune signal is generated to confirm the presence of an ongoing or a past viral infection (Fig 1). So far in the US, four serological test kits get authorized for emergency use by FDA and they are summarized in Table 1 for your reference.
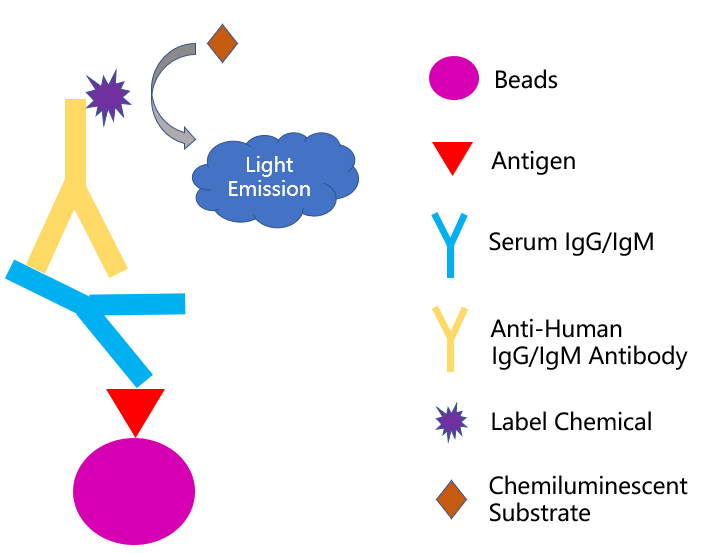
Fig 1. Principle of chemiluminescence-immunoassay method

Table 1 Antibody test kit manufacturers and commercial laboratories authorized for emergency use by FDA
As the importance of the serological test being noticed, a lot of studies are going on to evaluate these test kits. Recent work was shared online by a team from the Southern University of Science and Technology. [1] They developed a serological test for the diagnosis of SARS-Cov-2 using the chemiluminescence immunoassay method, which was explained in detail previously (Fig.1). The recombinant nucleocapsid protein of SARS-CoV-2 was successfully expressed from E. coli. and then immobilized to the magnetic beads to capture the IgM and IgG induced by SARS-CoV-2.
29 healthy individuals, 51 tuberculosis patients, and 79 SARS-CoV-2 patients were randomly enrolled in this study.
In the IgM testing, the average RLU (relative light units) of the patient group was higher than the control groups, consisting of health cohort and tuberculosis patients. When it comes to the IgG testing, the difference is even more dramatic as you can see in Fig.2. A Receiver Operating Characteristic (ROC) curve was obtained based on all the RLUs values. According to the ROC curve, both IgM and IgG testing have predictive values with a cutoff setting for IgM (RLU 162296) and IgG (RLU 336697), in which the calculated sensitivity and specificity for IgM were 82.28% (65/79)and 81.25% (15/80), and 82.28% (65/79) and 97.5% (2/80) for IgG, respectively.
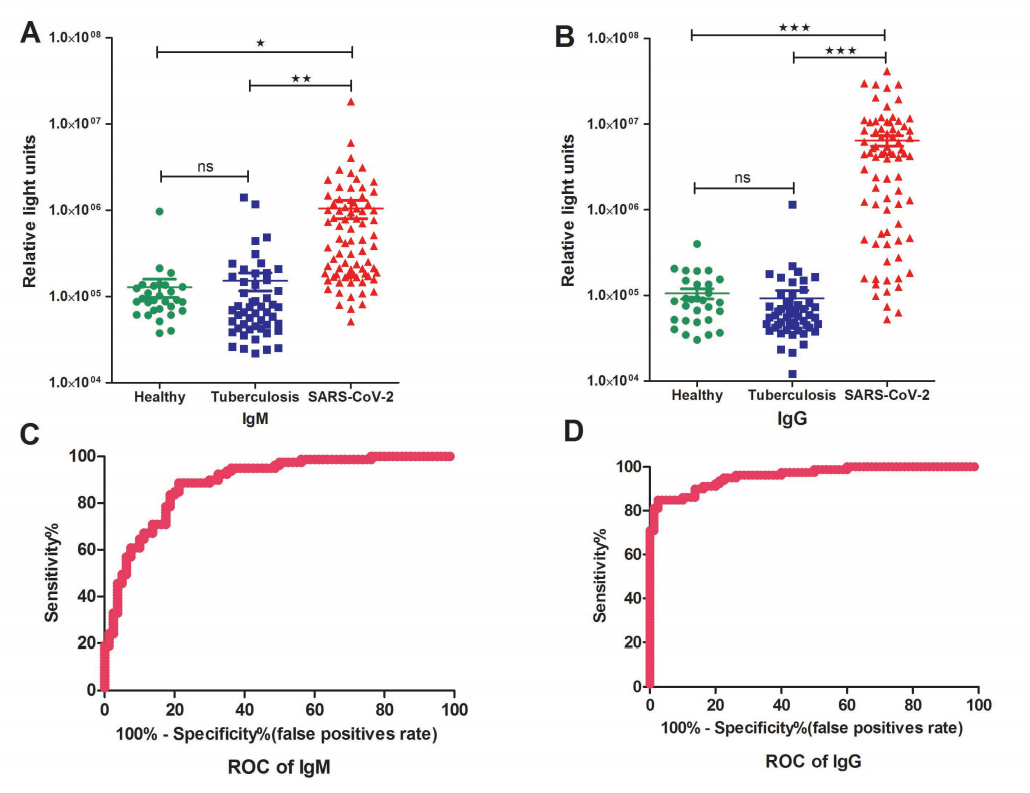
Fig 2. Detections and measurements of the SARS-CoV-2 IgM and IgG antibody in healthy people, tuberculosis patients and SARS-CoV-2 confirmed patients using the chemiluminescence immunoassay method (A and B). The average results were expressed as mean ± SEM of all individuals. Receiver Operating Characteristic curves for IgM (C) and IgG(D) were obtained based on the RLU for the SARS-CoV-2 patient group and the control group consisting of healthy cohorts and tuberculosis patients.
(Source: Evaluations of serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak.)
No statistically significant difference was observed between IgM and IgG testing results despite the onset time or gender. But they found a significantly lower detection rate in both IgM and IgG testing for individual groups younger than 18 years old, which was likely associated with no or less production of antibodies in them yet. They also compared with a commercially available ELISA kit and found their chemiluminescence method had significantly higher sensitivity. The sensitivity difference may be partially attributed to the different serum amounts for the incubation step. Although, the intrinsic method difference can also contribute.
A well-developed serological test with high sensitivity and specificity is sufficient to become a diagnostic tool for COVID-19. As an important supplement to the molecular test, the serological test should gain more attention.
Nucleocapsid protein is an essential part of SARS-CoV-2. Due to its high immunogenicity, the nucleocapsid protein is always chosen to capture IgG/IgM in the COVID-19 serological kits. As the critical component in the serological test kit, the recombinant nucleocapsid protein of SARS-CoV-2 has a significant effect on the sensitivity and specificity.
Reference:
Dachuan Lin et al. Evaluations of serological test in the diagnosis of 2019 novel coronavirus (SARS-CoV-2) infections during the COVID-19 outbreak.
medRxiv preprint doi: https://doi.org/10.1101/2020.03.27.20045153
ACROBiosystems has developed different types of N protein. The binding activity of N protein with anti-N protein antibody was verified by ELISA, with a high sensitivity of 0.02 ng/mL.
Product:SARS-CoV-2 (COVID-19) Nucleocapsid protein, His Tag (C-terminus)
The sensitivity of N protein (His tag at the C-terminus) with anti-N protein antibody was 0.02 ng/mL as verified by ELISA.
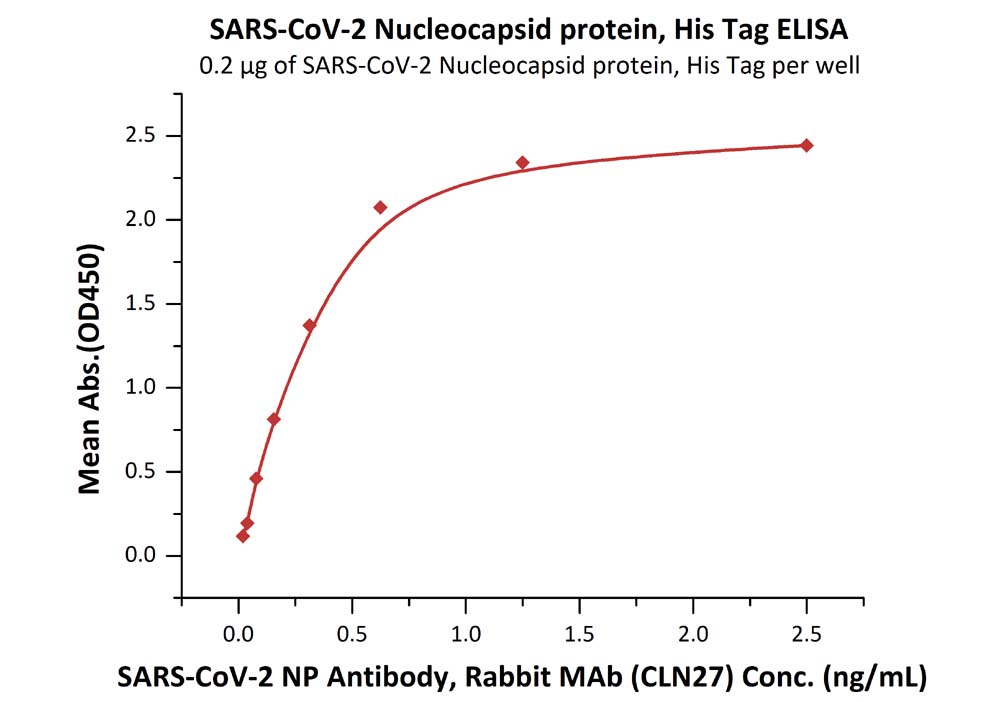
Immobilized SARS-CoV-2 Nucleocapsid protein, His Tag (Cat. No. NUN-C5227) at 2 μg/mL (100 μL/well) can bind SARS-CoV-2 NP Antibody, Rabbit MAb (CLN27) with a linear range of 0.02-0.6 ng/mL.
Product:SARS-CoV-2 (COVID-19) Nucleocapsid protein, His Tag (N-terminus)
The sensitivity of N protein (His tag at the N-terminus) with anti-N protein antibody was 0.02 ng/mL as verified by ELISA.
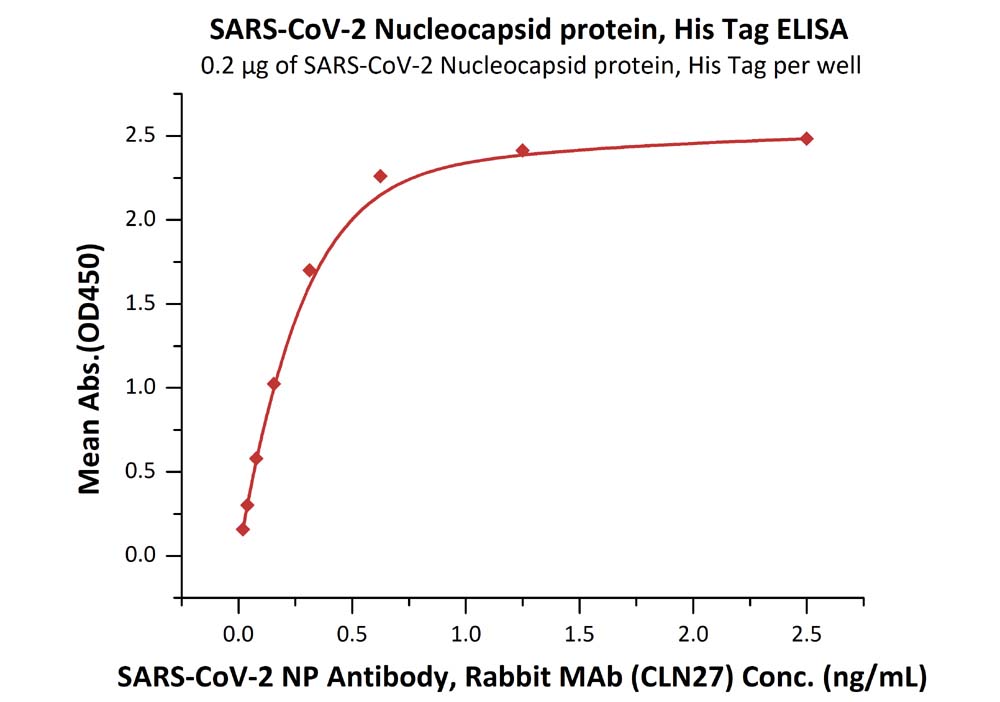
Immobilized SARS-CoV-2 Nucleocapsid protein, His Tag (Cat. No. NUN-C51H9) at 2 μg/mL (100 μL/well) can bind SARS-CoV-2 NP Antibody, Rabbit MAb (CLN27) with a linear range of 0.02-0.3 ng/mL.
Product:Biotinylated SARS-CoV-2 (COVID-19) Nucleocapsid protein, His,Avitag™
The sensitivity of biotinylated N protein with anti-N protein antibody was 0.02 ng/mL as verified by ELISA.
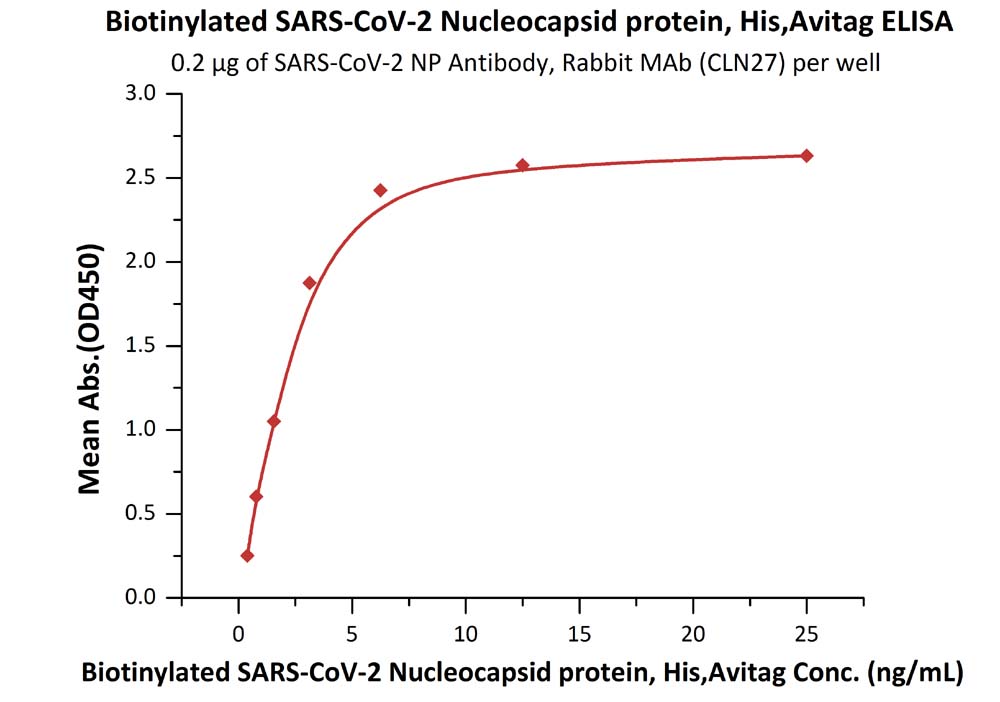
Immobilized SARS-CoV-2 NP Antibody, Rabbit MAb (CLN27) at 2 μg/mL (100 μL/well) can bind Biotinylated SARS-CoV-2 Nucleocapsid protein, His,Avitag (Cat. No. NUN-C81Q6) with a linear range of 0.4-3 ng/mL.
| Molecule | Cat.No. | Species | Host | Product Description | |
| Nucleocapsid protein | NUN-C51H9 | SARS-CoV-2 | E.coli | SARS-CoV-2 (COVID-19) Nucleocapsid protein, His Tag | |
| NUN-C5227 | SARS-CoV-2 | HEK293 | SARS-CoV-2 (COVID-19) Nucleocapsid protein, His Tag | ||
| NUN-C81Q6 | SARS-CoV-2 | E.coli | Biotinylated SARS-CoV-2 (COVID-19) Nucleocapsid protein, His,Avitag™ |
This web search service is supported by Google Inc.
