
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































Over 40 years ago, the World Health Organization (WHO) officially declared that smallpox had been eradicated. As we progress deeper into the vaccination era with widespread vaccination programs held across the globe, several other diseases are no on the precipice of being eradicated. Now the question becomes, is it possible for cervical cancer to be next?
HPV is a unique case for vaccinations, since the virus itself has accounted for over 95% of cervical cancer in women, the fourth most common type of cancer. With the release of an HPV vaccine in 2006 and the first patients beginning to reach the average age of cervical cancer onset, the success of HPV vaccines is becoming more and more clear.
“Vaccines are bringing the dream of eliminating cervical cancer within reach.”
- General Tedros Adhanom Ghebreyesus, WHO director.
Within the US, the introduction of vaccine recommendations by the CDC alongside with regular screening has led to a significant decline in cervical cancer cases. According to the American Cancer Society, among women from 20 to 24, cervical cancer incidence rates declined over 65%.
The WHO Strategic Advisory Group of Experts (SAGE) have begun to push doctors across the globe to change their advice regarding HPV vaccines, paving the way for the prevention of cancer through vaccinations. However, one might point out that HPV vaccines have been publicly available for a while. Why hasn’t cervical cancer been eliminated yet?
The introduction of HPV vaccine into immunization programs has slowed in recent years with various factors coming into play, such as the growing anti-vaccination movement, limitations in resource, or narrow indications-of-use in existing HPV vaccines. These factors are exemplified by a low global coverage of 13% -- a far cry from the 90% target set by WHO.
However, a major issue stems from the recommended three-dose schedule. Countries with the highest incidence rates of cervical cancer, and by extension, HPV infections, generally have more limited resources and more regions with limited medical access. Vaccines that require more than one dose are not well-adopted and almost always end in patient non-compliance.
To achieve the lofty goals that WHO has challenged us with, HPV development should be focused on making the vaccine cheaper, less resource-intensive, and easier to administer. This includes expanding the indications-of-use into different age-groups to cover women who are outside of the current age group and lacked access to HPV vaccines.

Similarly, a one-dose schedule would greatly improve vaccination rates by eliminating the logistical or cost challenges in implementing them into vaccination programs. This, however, requires further cohort studies to ensure that the change in dosing recommendations would maintain its current efficacy.
The success of HPV vaccines does not mean that our current vaccines are perfect. Each country offers different vaccination options, which means that the feasibility of implanting a one-dose schedule different for everyone. Developing a sustainable, one-dose HPV vaccine that has been comprehensively evaluated is a significant challenge that might take years, but also might be the right path towards eliminating cervical cancer globally.
As with most virus research, a major bottleneck is the difficulty in cultivating viruses in vitro. Despite the significant safety concerns for scientists and the laboratory involved, the actual virus is a critical component in neutralizing antibody titer studies. This is the key evaluation study that counts the number of antibodies produced by the immune system, a direct indicator as to the efficacy of new and potential vaccines.
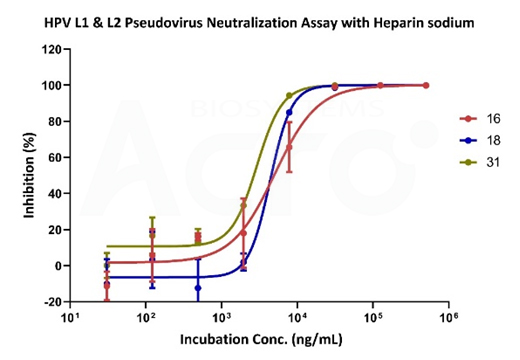
Figure X. Neutralizing data of sodium heparin using various HPV-subtype pseudoviruses.
However, most laboratories are not equipped with the proper equipment to store and manipulate viruses. That means using the actual virus is out of the question. Pseudoviruses, on the other hand, are virus particles that express the same proteins on their membrane surfaces like actual viruses. They are designed to mimic viral entry into the host cell without the autonomous replication ability and pathogenic genetic sequences.
With the availability of HPV pseudoviruses, evaluation of HPV vaccines can be performed more frequently, helping drive along the possibility of developing a sustainable, one-dose HPV vaccine and eliminating cervical cancer.
ACROBiosystems is always monitoring and tracking the development of various viruses and infectious diseases around the globe to help quickly offer a wide array of products designed to accelerate the development of vaccines and other anti-viral therapeutics under our ViruStop brand. This includes a wide-ranging virus antigen bank such as SARS-COV-2, influenza, Nipah, Ebola, and many others.
Under ViruStop, we offer a wide array of HPV pseudoviruses, including subtypes 6, 11, 16, 18, 31, 33, 45, 52, and 58. Pseudovirus services are also offered, including custom reporter pseudovirus development (both enveloped and non-enveloped viruses) as well as other technical evaluation service such as screening, evaluation of anti-HPV drugs, neutralizing antibody assays, and other vaccine efficacy assessments. Explore our ViruStop platform and let ACROBiosystems help accelerate your infectious disease research or vaccine development process!
This web search service is supported by Google Inc.











