> Insights > GMP-grade DLL4: A Feeder-cell Alternative for Scale-up iPSC Manufacturing 
Cellular immunotherapy, exemplified by Chimeric Antigen Receptor (CAR)-T cell therapy, has proven highly effective in treating various hematologic malignancies. The landscape of CAR-T therapy has evolved significantly, with multiple drugs secured regulatory approval for commercialization. Despite these successes, significant challenges persist in mainstream autologous CAR-T therapy that still need to be addressed, mainly the complexities of acquiring patient T cells, prolonged manufacturing and difficulties in scale-up manufacturing processes.
Successful CAR-T therapy production often hinges on securing robust supply of patient’s T cells. In response to this challenge, researchers have identified an alternative cell sources for T cells, including embryonic stem cells (ESCs), and most notably, induced pluripotent stem cells (iPSCs). In recent years, iPSCs have emerged as a promising solution due to their distinctive self-renewal properties and the capacity for gene editing. iPSCs offer a renewable source of T cells, establishing an "infinite supply" platform for cell therapy. This strategic utilization of iPSCs addresses critical bottlenecks in conventional approaches, marking a significant advancement in the field of cellular immunotherapy.
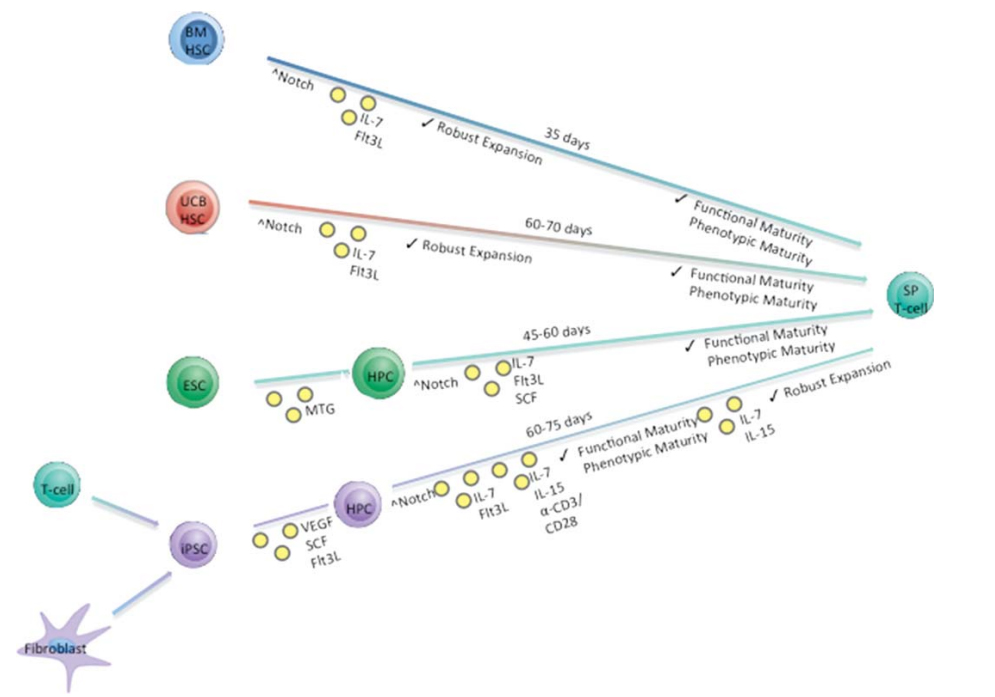
Simplified in vitro differentiation schema describing the approximate time needed to generate mature SP T cells from four starting progenitor cell populations. Stem Cells. 2015;33(11):3174-3180.1
The conventional approach to differentiate iPSCs into T cells involves co-culturing of iPSCs from healthy donor with murine bone marrow derived stromal cell lines, OP9. This facilitates the generation of CD34+ hematopoietic progenitor cells (HPCs). Subsequently, these HPCs undergo enrichment with specific growth factors (SCF, Flt3, IL-7, IL-3, and TPO), along with co-culture with OP9 cells overexpressing either DLL1 or DLL4. This effectively initiates the T-cell differentiation, leading to the successful generation of iPSC-induced differentiated T cells.
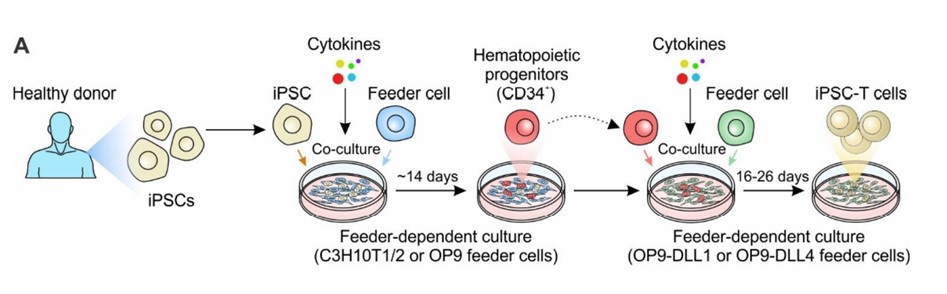
Generation of iPSC-derived T cell-based cell therapy. Cancers (Basel). 2022;14(9):2266.2
The physical interaction between Notch receptors and their ligands plays vital roles in the development of multiple cell lineages, extending beyond T cells. In the context of T cell production from iPSCs, this interaction is prominently orchestrated by Notch1 and DLL4. The interplay unfolds through the interaction between the Notch1 receptor expressed on hematopoietic progenitor cells (HPCs) and DLL1/DLL4 expressed on OP9-DL cells. This intercellular dialogue initiates a cascade of precisely regulated steps, ultimately resulting in the differentiation of iPSCs into the T cell lineage.
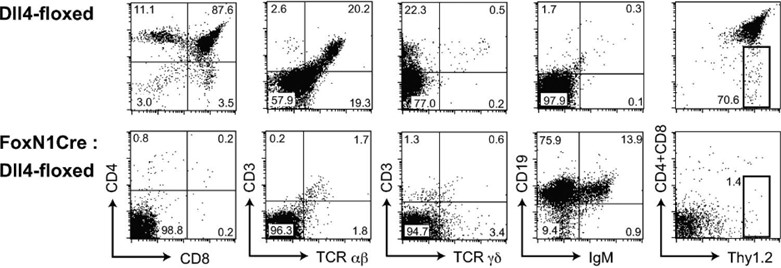
No T cells, but aberrant B cell accumulation, in the thymus with Dll4-null epithelial cells,J Exp Med. 2008;205(11):2507-2513.3
Both DLL1 and DLL4 have demonstrated their capability to support T cell derived iPSC, iPSC-T. To delve deeper into the comparative effectiveness of these DLL variants in inducing HPCs into the T cell lineage in vitro, a series of OP9 cells expressing either DLL1 or DLL4 with varying expression levels (low/medium/high) were used to elucidate the induction of HPCs into T cells. Surprisingly, the results indicated that, despite the lower cell surface protein level of DLL4-HA on OP9-DL4hi and OP9-DL4med cells compared to DLL1-HA on OP9-DL1hi and OP9-DL1med cells, feeder cells expressing DLL4 exhibited a higher efficacy in supporting T cell generation. In summary, this study underscores DLL4 as a more effective inducer of HPCs into the T cell lineage compared to DLL1.
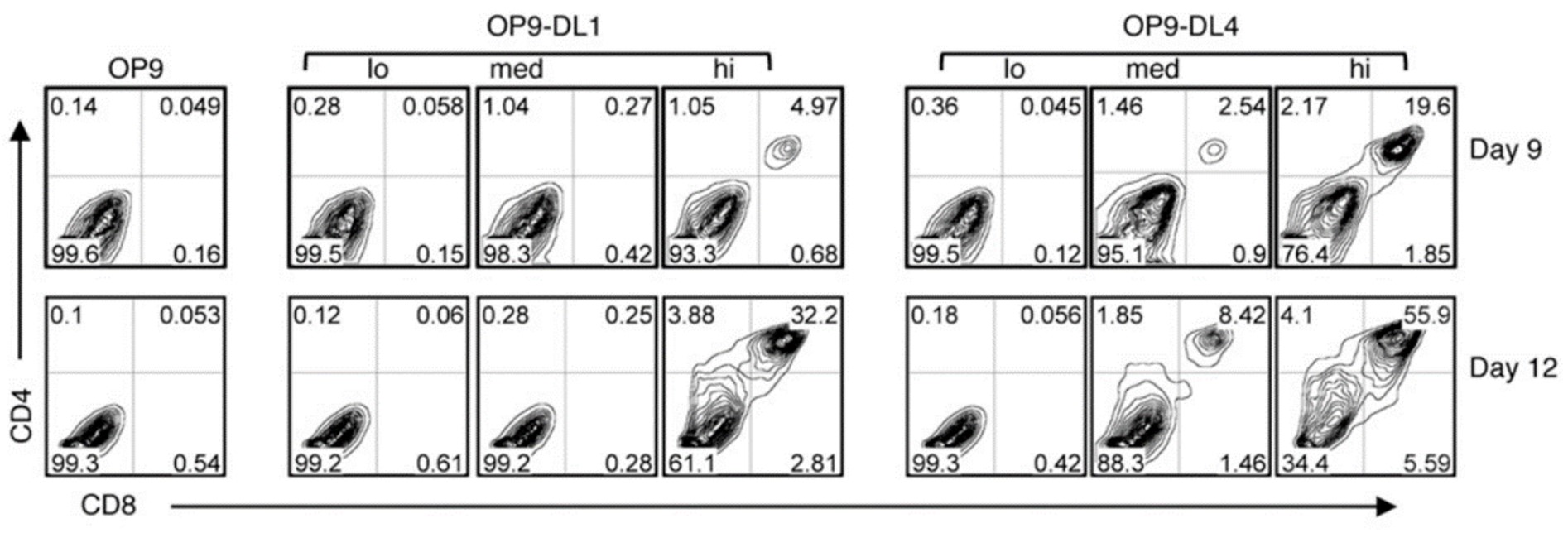
Comparison of T cell development from HPCs cultured with OP9-DL1 (lo/med/hi) and OP9-DL4 (lo/med/hi) cell lines. J Immunol. 2010;185(2):867-876.(re 4)
In the classic method, feeder cells like OP9 are crucial components in iPSC-T induction and differentiation. However, reliance on mouse-derived stromal cell lines and certain undefined extracts introduces the risk of cross-species contamination, posing challenges for stringent quality control in clinical applications. To overcome these limitations, feeder-cell free cultures utilizing recombinant DLL4 protein have emerged as the predominant solution for clinical-grade iPSC-T production. This involves the use of plates combined with DLL4 and adhesion molecules like VCAM-1, enhancing the clinical translational potential. Notably, the Zúñiga-Pflücker group in the University of Toronto have advanced the field by developing feeder-cell free in vitro systems employing DLL4 microbeads, a strategy that significantly improves support for large-scale suspension culture of iPSCs.5
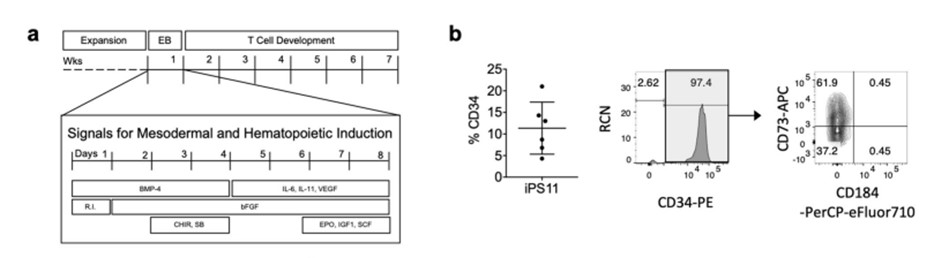
T cell development from human pluripotent stem cells using DLL4-μbeads, Nat Commun. 2021;12(1):5023.5
As iPSCs emerge as a promising and versatile cell source for CAR-T therapy, scaling up cellular differentiation into iPSC-T cells is a crucial step in overcoming manufacturing challenges plaguing current CAR-T therapies. Despite the prevalence of feeder cells for differentiation, feeder cells pose a major risk to the safety of biologics especially during scale-up and clinical applications. With the introduction of GMP-grade DLL4, the addition of individual recombinant factors can replace feeder cells with recent research achieving sufficient numbers for safety evaluations in clinical trials. This is an indispensable advancement in overcoming challenges associated with autologous cell therapy. As such, the availability of GMP-grade DLL4 not only addresses challenges related to T cell scarcity in patients but also broadens the scope of potential beneficiaries in cell therapy. In the dynamic landscape of cancer treatment, with cellular immunotherapy at the forefront, this breakthrough in iPSC-T cell manufacturing holds tremendous potential in ushering in a new era of therapeutic possibilities.
ACROBiosystems is dedicated to developing high-quality reagents and raw materials for use in CGT clinical stages. With a robust GMP quality management system, we are pleased to announce the release of our GMP-grade recombinant DLL4 protein, which demonstrates exceptional activity and safety. This protein is ideal for supporting the feeder-cell free approach to iPSC-to-T cell differentiation, particularly for large-scale clinical manufacturing of iPSCs.
1. Simplified in vitro differentiation schema describing the approximate time needed to generate mature SP T cells from four starting progenitor cell populations. Stem Cells. 33(11):3174-3180 (2015)
2. Yang Zhou, Miao Li, Kuangyi Zhou, James Brown, Tasha Tsao, Xinjian Cen, Tiffany Husman, Aarushi Bajpai, Zachary Spencer Dunn, and Lili Yang. Engineering Induced Pluripotent Stem Cells for Cancer Immunotherapy. Cancers (Basel). 14(9): 2266 (2022).
3. Katsuto Hozumi, Carolina Mailhos, Naoko Negishi, Ken-ichi Hirano, Takashi Yahata, Kiyoshi Ando, Saulius Zuklys, Georg A. Holländer, David T. Shima, Sonoko Habu. Delta-like 4 is indispensable in thymic environment specific for T cell development. J Exp Med. 205 (11): 2507–2513(2008)
4. Mahmood Mohtashami; Divya K. Shah; Hiroshi Nakase; Korosh Kianizad; Howard T. Petrie; Juan Carlos Zúñiga-Pflücker. Direct Comparison of Dll1- and Dll4-Mediated Notch Activation Levels Shows Differential Lymphomyeloid Lineage Commitment Outcomes. J Immunol.185 (2): 867–876 (2010).
5. Trotman-Grant A.C., Mohtashami M., De Sousa Casal J., Martinez E.C., Lee D., Teichman S., Brauer P.M., Han J., Anderson M.K., Zuniga-Pflucker J.C. DL4-mubeads induce T cell lineage differentiation from stem cells in a stromal cell-free system. Nat. Commun. 12:5023. (2021).
This web search service is supported by Google Inc.
