Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Insights > COVID-19 anti-nucleocapsid antibodies verified with inactivated virus samples The health-care company Abbott transformed the testing landscape of the United States. On August 27, they announced that it had received FDA authorization to distribute a new type of test. The tests will cost just $5 apiece. The Trump administration announced that it would be purchasing 150 million of these tests from the company. For comparison, states have reported fewer than 75 million tests total over the past six months.
Abbott’s test is a kind of antigen test. Compared to the PCR test, the antigen test is convenient, quicker, and low-cost. Compared to the antibody test, the antigen test can be used to detect people who carry the virus but don’t show symptoms, which is not limited to the window of onset.
The antibody pairs are the critical reagents for antigen test kits. Antibody pairs with good quality can help increase the specificity and sensitivity. Potentially, the sensitivity of the antigen test could match the good sensitivity of the PCR test.
However, for the antigen test development, the current dilemma is how to choose a sample for the assay development. There is no doubt that the best sample is the patient's nasopharyngeal swab. But due to the biosafety reason, it is not easy to get access to patient samples in the early discovery stage. So, most of the scientists use recombinant antigen proteins as spiked in samples for the assay development. The deficiency of this method is that the recombinant proteins may not best represent the structure of the virus. Additionally, the lysis of the swab samples will cause unwanted breakage of the proteins, which is the recombinant protein won’t be able to replicate. In summary, the recombinant protein is not sufficient enough when screening for the antibody pairs.
Case study:
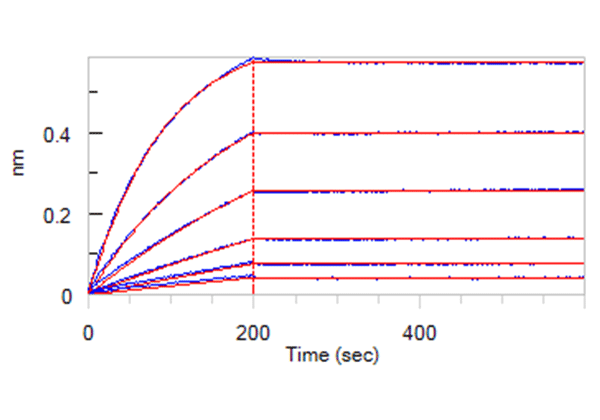
Two different antibody pairs were tested using recombinant proteins and inactivated virus. As we can see from figure 1 below, the sensitivities are different.
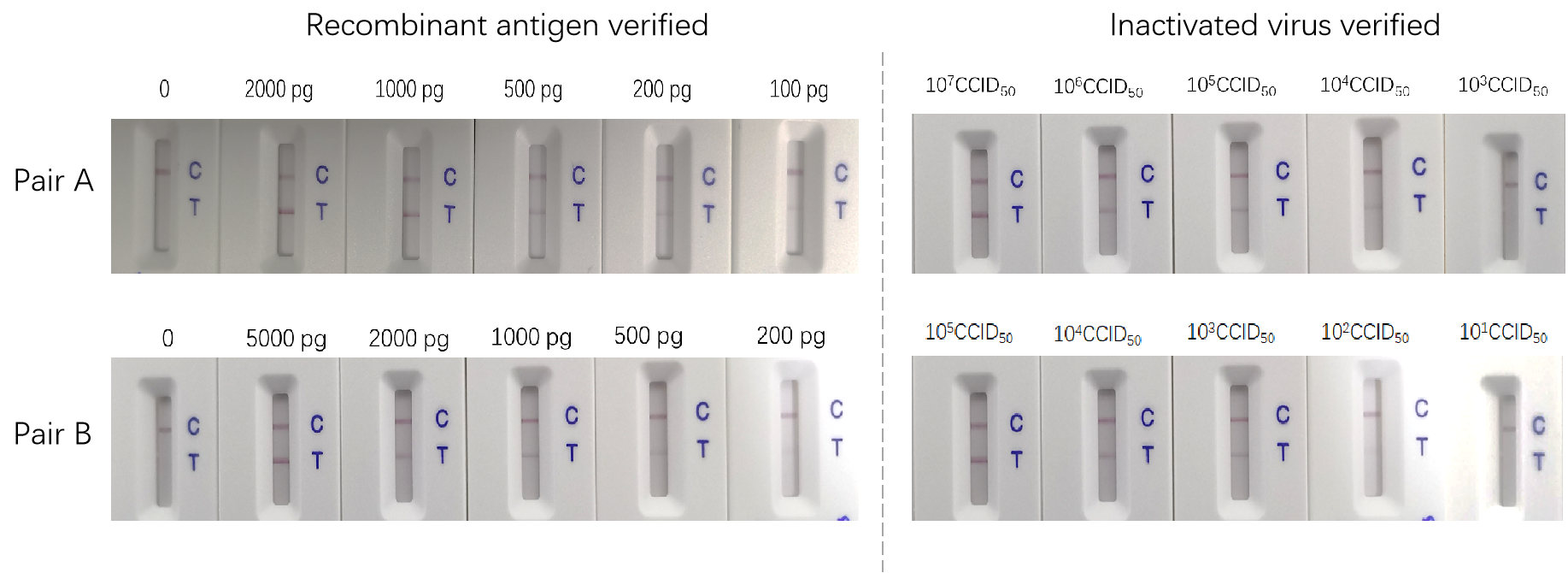
Figure 1. Antigen tests using recombinant protein and inactivated virus, separately
The most reliable way to evaluate your antigen test is using inactivated virus for the assay development. In order to support the IVD product development, ACRO developed a series of high-quality recombinant protein reagents, especially the antibody pairs. The sensitivity of the sandwich ELISA can be as low as 12 pg/mL in the verification study using recombinant antigens. In addition, ACRO’s antibody pairs have been verified using inactivated virus samples. The titer, which was determined using our LFA assay, is as low as 100 CCID50.
These antibodies were recommended for antigen detection by double antibody sandwich.
>>> Virus lysis buffer is available for antigen detection kit development. Request
>>> If you have any bulk order inquiry, please feel free to click here.
Verification study using inactivated virus
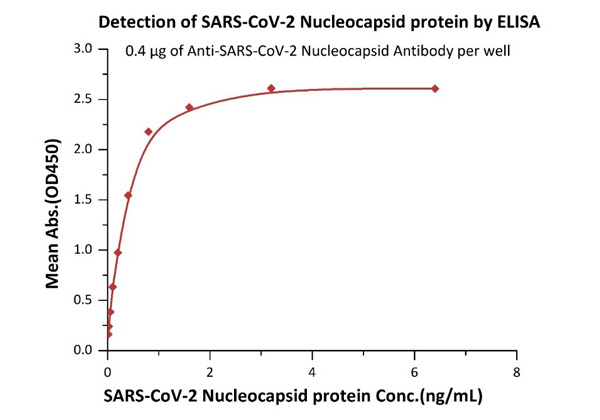
Fig 1 Immobilized Anti- SARS-CoV-2 Nucleocapsid antibody, mouse Mab (Cat. No. NUN-S46) at 4 μg/mL, add increasing concentrations of SARS-CoV-2 Nucleocapsid protein and then add Biotinylated Anti- SARS-CoV-2 Nucleocapsid antibody, mouse Mab (Cat. No. NUN-S47). Detection was performed using HRP-conjugated streptavidin with sensitivity of 12 pg/mL.
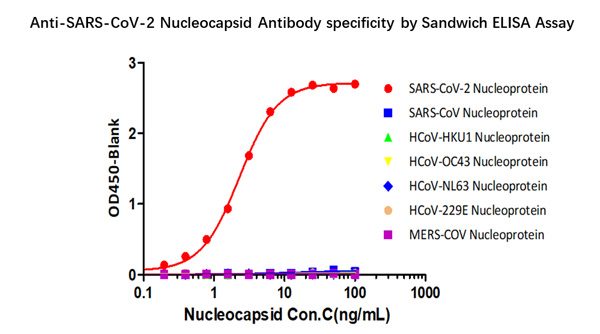
Fig 3 Demonstration of the specificity of Anti-SARS-CoV-2 N antibody pairs (Cat. No. NUN-S46 & Cat. No. NUN-S47) to the Nucleocapsid protein.
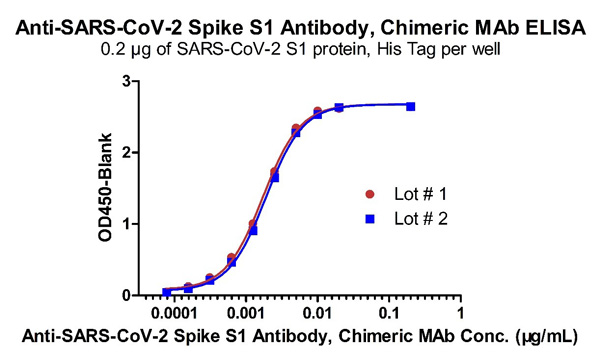
Fig 4 Demonstration of the Batch to Batch Consistency of Anti-SARS-CoV-2 Spike S1 Antibody, Chimeric Mab (Cat. No. S1N-M122) to the S1 protein.
This web search service is supported by Google Inc.