
Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Diagnostic Kits of SARS-CoV-2 1. Neutralizing Antibody Detection Kits (ELISA),
2. Neutralizing Antibody Detection Kits (LFA),
3. SARS-CoV-2 Antigen Rapid Detection Kit (LFA),
4. ......

Anti-SARS-CoV-2 Neutralizing Antibody Detection Kit (Spike RBD) developed by ACRODagnostics is based on the competitive ELISA method, intended for the detection of SARS-CoV-2 neutralizing antibody that blocks the interaction between the receptor-binding domain of the viral spike glycoprotein (RBD) and the ACE2 cell surface receptor in human serum. SARS-CoV-2 Neutralizing antibody is an important marker for accessing the effectiveness of the novel coronavirus vaccines. The reagent is for neutralizing antibodies detection in samples from individuals after vaccine injection or removed from COVID-19.
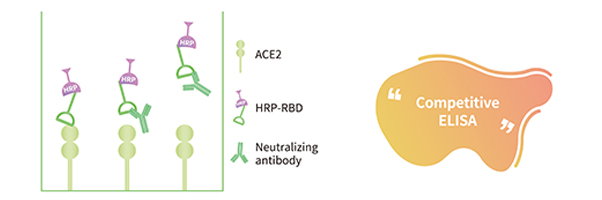
The microplate is pre-coated with Human ACE2 Protein. The presence of neutralizing antibodies in samples will compete with ACE2 for HRP- SARS-CoV-2 Spike RBD binding. The intensity of assay signal decrease proportionally to the presence of Anti-SARS-CoV-2 Neutralizing Antibody.
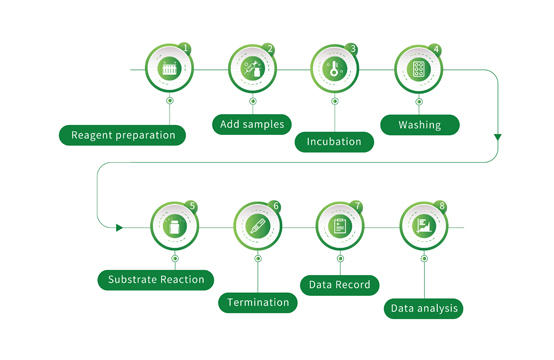
The SARS-CoV-2 Neutralizing Antibody Detection Kit can be used for the qualitative detection of SARS-CoV-2 neutralizing antibodies that blocks the interaction between the receptor-binding domain of the viral spike glycoprotein (RBD) and the ACE2 cell surface receptor in human serum, plasma or whole blood. The reagent is for neutralizing antibodies detection in samples from individuals after vaccine injection or removed from COVID-19.
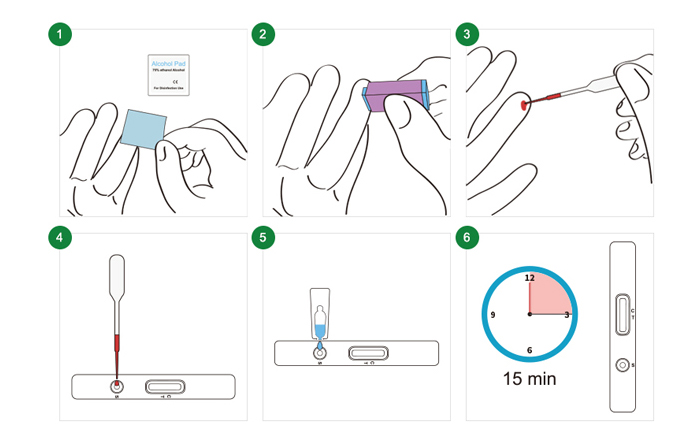
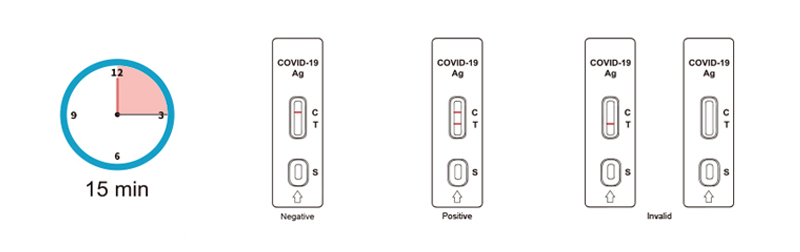
Transfer 20μL of serum or plasma vertically into the specimen well (S) of the test strip, then add 100 μL of sample diluent to the specimen well, and timer followed.
Use blood lancet to prick the fingertip and squeeze the finger to bleed. Use the dropper to take 20μL of fingertip blood (about 1 drop) and add into the specimen well (S) of the test strip. Open the buffer tube by twisting off the top, hold the buffer bottle vertically and 1 cm above the buffer well, add all the sample diluent (Vials with 100 µL) into the specimen well (S) of the test strip.
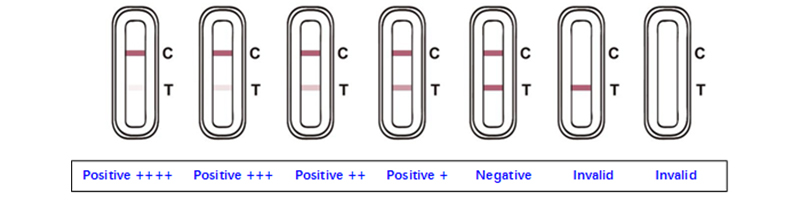
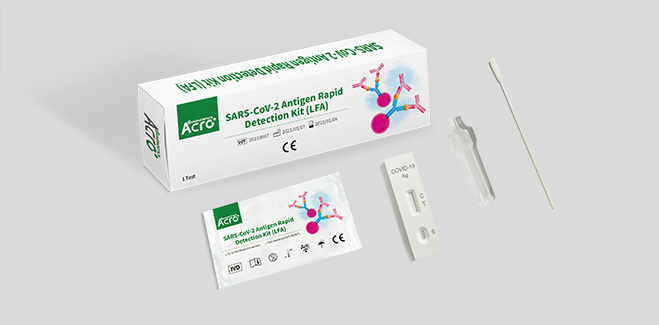
SARS-CoV-2 Antigen Rapid Test Kit (Colloidal Gold) is a lateral flow immunoassay for the qualitative detection of SARS-COV-2 antigen (nucleocapsid protein) in upper respiratory samples with nasal swabs or saliva during the acute phase of infection. An uncut sheet format is available.
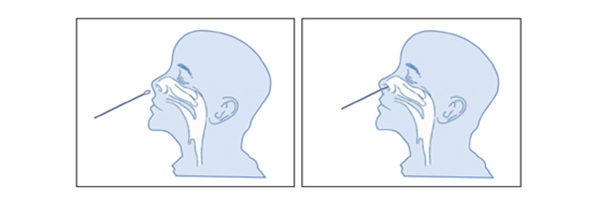
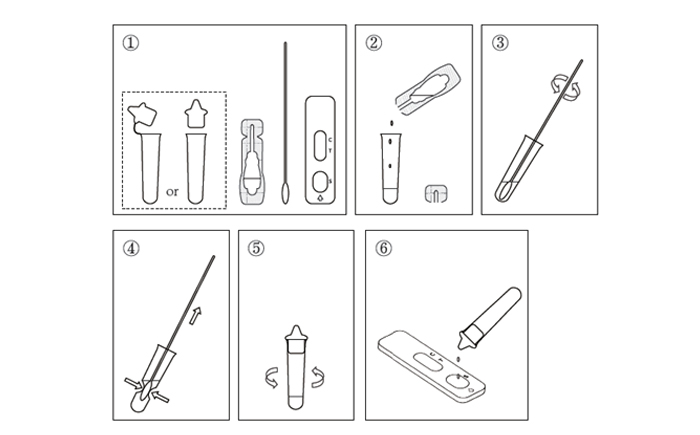
This web search service is supported by Google Inc.








