 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| MME-H521b | Human | Human Neprilysin / MME / CD10 Protein, Tag Free (active enzyme) |  |

|
|
| MME-H526a | Human | Human Neprilysin / MME / CD10 Protein, Fc Tag |  |
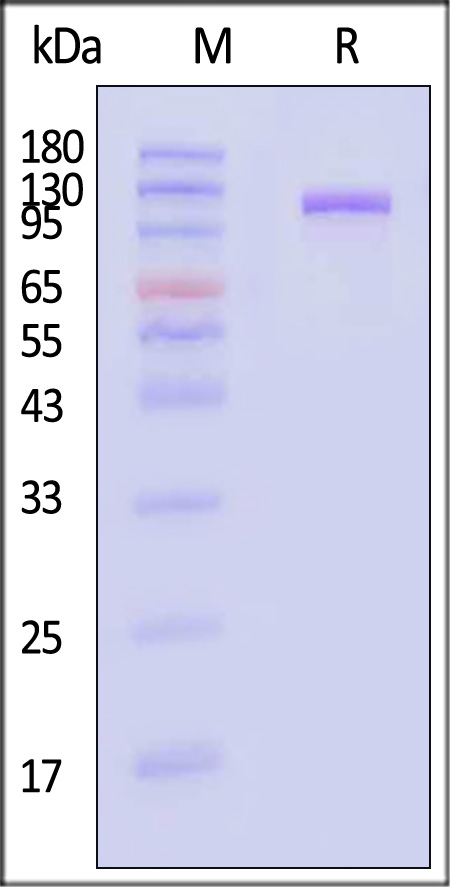
|
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Sacubitril/Valsartan Sodium Hydrate | LCZ-696; LCZ-696A | Approved | Novartis Pharma Ag | Entresto, 诺欣妥, 科畅欣, Neparvis, ENTRESTO SPRINKLE, Enresuto, Enrest | United States | Heart Failure | Novartis Pharmaceuticals Corp | 2015-07-07 | Breast Neoplasms; Heart Failure, Systolic; Peripheral Arterial Disease; Ventricular Dysfunction, Left; Lymphoma; Obesity; Cardiovascular Diseases; Hepatic Insufficiency; Essential Hypertension; Erectile Dysfunction; Hypertension; Coronavirus Disease 2019 (COVID-19); Postmyocardial Infarction Syndrome; Heart Diseases; Cardiomyopathy, Hypertrophic; Myocardial Infarction; Renal Insufficiency; Heart Failure | Details |
| Racecadotril | Tiorfan; Hidrasec | Approved | Bioprojet Pharma | France | Diarrhea | Bioprojet Pharma | 1993-03-23 | Diarrhea, Infantile; Diarrhea | Details | |
| Sacubitril/Valsartan Sodium Hydrate | LCZ-696; LCZ-696A | Approved | Novartis Pharma Ag | Entresto, 诺欣妥, 科畅欣, Neparvis, ENTRESTO SPRINKLE, Enresuto, Enrest | United States | Heart Failure | Novartis Pharmaceuticals Corp | 2015-07-07 | Breast Neoplasms; Heart Failure, Systolic; Peripheral Arterial Disease; Ventricular Dysfunction, Left; Lymphoma; Obesity; Cardiovascular Diseases; Hepatic Insufficiency; Essential Hypertension; Erectile Dysfunction; Hypertension; Coronavirus Disease 2019 (COVID-19); Postmyocardial Infarction Syndrome; Heart Diseases; Cardiomyopathy, Hypertrophic; Myocardial Infarction; Renal Insufficiency; Heart Failure | Details |
| Racecadotril | Tiorfan; Hidrasec | Approved | Bioprojet Pharma | France | Diarrhea | Bioprojet Pharma | 1993-03-23 | Diarrhea, Infantile; Diarrhea | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Dexecadotril | Phase 3 Clinical | Bioprojet Pharma | Gastrointestinal Diseases | Details | |
| Debio-0827 | PL-37; Debio-0827 | Phase 2 Clinical | Pharmaleads | Pain | Details |
| STR-324 | STR-324 | Phase 2 Clinical | Shanghai Pasteur Institute Of Chinese Academy Of Sciences | Acute Pain | Details |
| Sacubitril | AHU-377 | Phase 2 Clinical | Novartis Pharma Ag | Heart Failure; Cardiomyopathy, Hypertrophic; Insulin Resistance; Hypertension; Metabolic Diseases; Essential Hypertension; Cardiovascular Diseases; Diabetes Mellitus | Details |
| QR-12000 | QR-12000 | Phase 2 Clinical | Wuhan Createrna Science and Technology Co Ltd | Hypertension; Essential Hypertension | Details |
| Dexecadotril | Phase 3 Clinical | Bioprojet Pharma | Gastrointestinal Diseases | Details | |
| Debio-0827 | PL-37; Debio-0827 | Phase 2 Clinical | Pharmaleads | Pain | Details |
| STR-324 | STR-324 | Phase 2 Clinical | Shanghai Pasteur Institute Of Chinese Academy Of Sciences | Acute Pain | Details |
| Sacubitril | AHU-377 | Phase 2 Clinical | Novartis Pharma Ag | Heart Failure; Cardiomyopathy, Hypertrophic; Insulin Resistance; Hypertension; Metabolic Diseases; Essential Hypertension; Cardiovascular Diseases; Diabetes Mellitus | Details |
| QR-12000 | QR-12000 | Phase 2 Clinical | Wuhan Createrna Science and Technology Co Ltd | Hypertension; Essential Hypertension | Details |
This web search service is supported by Google Inc.
