 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
Request a FREE Sample of our Fc gamma RI / CD64 Binding Kit !
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
| Project Name | Project Stage | Molecule Type | Host Species | Therapeutic Area | Indications |
| 5T4 mAb - 01 | Lead | Solid Tumor | Ovarian cancer,Lung cancer |
| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| CHEK-ATP176 | Human | HEK293/Human TPBG Stable Cell Line | |||
| TPG-R52H6 | Rat | Rat TPBG / 5T4 Protein, His Tag (MALS verified) |  |

|
|
| TPG-M5255 | Mouse | Mouse TPBG / 5T4 Protein, Fc Tag (MALS verified) |  |

|
|
| TPG-H82F6 | Human | Biotinylated Human TPBG / 5T4 protein, Fc,Avitag™ (MALS verified) |  |

|
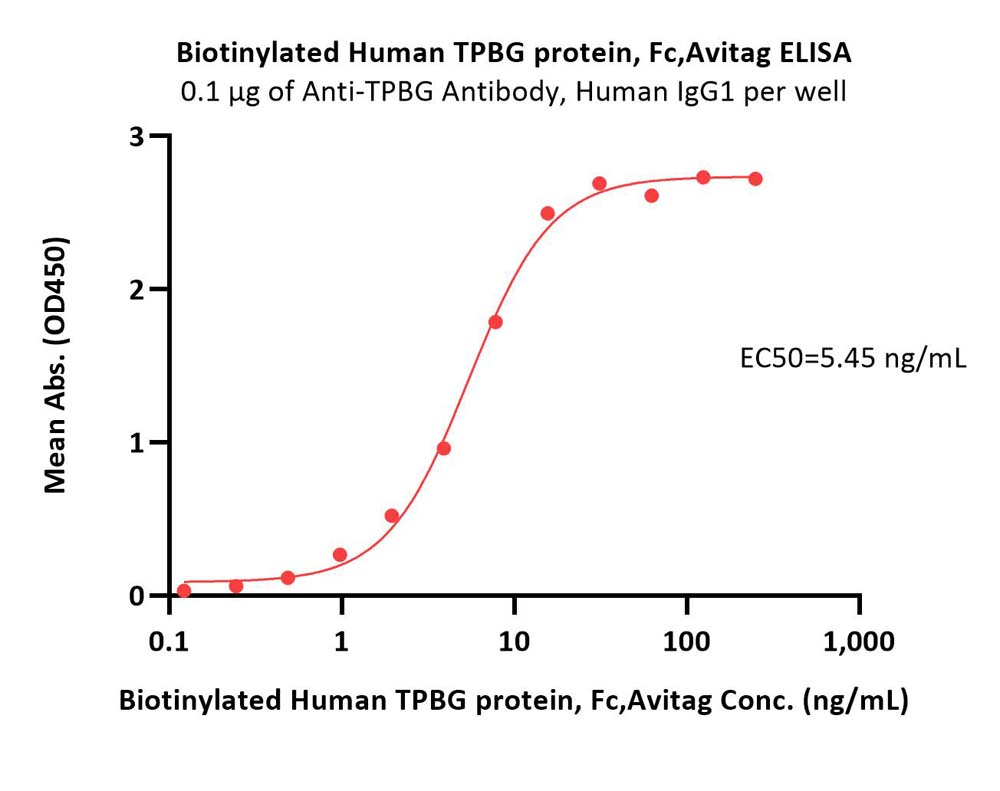
|
| TPG-C52H3 | Cynomolgus / Rhesus macaque | Cynomolgus / Rhesus macaque TPBG / 5T4 Protein, His Tag |  |

|

|
| TPG-M52H3 | Mouse | Mouse TPBG / 5T4 Protein, His Tag (MALS verified) |  |

|
|
| TPG-H5253 | Human | Human TPBG / 5T4 Protein, Fc Tag (MALS verified) |  |

|

|
| TPG-H52E5 | Human | Unconjugated Human TPBG / 5T4 Protein, His,Avitag™ |  |

|

|
| TPG-H82Eb | Human | Biotinylated Human TPBG / 5T4 Protein, His,Avitag™ |  |

|

|

Expression analysis of human TPBG on HEK293/Human TPBG Stable Cell Line by FACS.
Cell surface staining was performed on HEK293/Human TPBG Stable Cell Line or negative control cell using anti-human TPBG antibody followed by staining with PE anti-human IgG antibody.

Immobilized Cynomolgus / Rhesus macaque TPBG, His Tag (Cat. No. TPG-C52H3) at 1 μg/mL (100 μL/well) can bind Anti-TPBG Antibody, Human IgG1 with a linear range of 0.1-4 ng/mL (QC tested).

The purity of Rat TPBG Protein, His Tag (Cat. No. TPG-R52H6) is more than 90% and the molecular weight of this protein is around 42-57 kDa verified by SEC-MALS.
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| Naptumomab estafenatox | TTS-CD3; ABR-217620 | Phase 2 Clinical | Active Biotech Ab | Carcinoma, Renal Cell; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Non-Small-Cell Lung | Details |
| ALG.APV-527 | ALG-APV-527; ADC-1016; ATOR-1016; ALG.APV-527 | Phase 2 Clinical | Aptevo, Alligator Bioscience Ab | Solid tumours; Neoplasms | Details |
| OXB-301 | OXB-301; SAR-109659 | Phase 2 Clinical | Oxford Biomedica | Ovarian Neoplasms; Carcinoma, Renal Cell; Prostatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Details |
| SYD-1875 | SYD-1875 | Phase 1 Clinical | Synthon Bv | Solid tumours | Details |
| ASN-004 | ASN-004 | Phase 1 Clinical | Asana Biosciences Llc | Solid tumours | Details |
| ACR-246 | ACR246; ACR-246 | Phase 1 Clinical | Hangzhou Adcoris Biopharmacy Co Ltd | Solid tumours | Details |
| FTL-008.16 | FTL-008.16; FTL008.16 | Phase 1 Clinical | Sound(Chengdu)Biopharmacauticals Co Ltd | Solid tumours | Details |
| IBR-854 | IBR854; IBR-854 | Phase 1 Clinical | Imbioray (Hangzhou) Biomedicine Co Ltd | Solid tumours; Neoplasms | Details |
| CBA-1535 | CBA-1535 | Phase 1 Clinical | Chiome Bioscience Inc | Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Mesothelioma; Carcinoma, Non-Small-Cell Lung | Details |
| Anti-5T4 CAR-raNK Cell Therapy | Phase 1 Clinical | Imbioray (Hangzhou) Biomedicine Co Ltd | Solid tumours | Details | |
| Prostate cancer vaccine(inactivated viral)(University of Oxford) | Clinical | University Of Oxford | Prostatic Neoplasms | Details | |
| Naptumomab estafenatox | TTS-CD3; ABR-217620 | Phase 2 Clinical | Active Biotech Ab | Carcinoma, Renal Cell; Pancreatic Neoplasms; Carcinoma, Transitional Cell; Carcinoma, Non-Small-Cell Lung | Details |
| ALG.APV-527 | ALG-APV-527; ADC-1016; ATOR-1016; ALG.APV-527 | Phase 2 Clinical | Aptevo, Alligator Bioscience Ab | Solid tumours; Neoplasms | Details |
| OXB-301 | OXB-301; SAR-109659 | Phase 2 Clinical | Oxford Biomedica | Ovarian Neoplasms; Carcinoma, Renal Cell; Prostatic Neoplasms; Breast Neoplasms; Colorectal Neoplasms; Peritoneal Neoplasms; Fallopian Tube Neoplasms | Details |
| SYD-1875 | SYD-1875 | Phase 1 Clinical | Synthon Bv | Solid tumours | Details |
| ASN-004 | ASN-004 | Phase 1 Clinical | Asana Biosciences Llc | Solid tumours | Details |
| ACR-246 | ACR246; ACR-246 | Phase 1 Clinical | Hangzhou Adcoris Biopharmacy Co Ltd | Solid tumours | Details |
| FTL-008.16 | FTL-008.16; FTL008.16 | Phase 1 Clinical | Sound(Chengdu)Biopharmacauticals Co Ltd | Solid tumours | Details |
| IBR-854 | IBR854; IBR-854 | Phase 1 Clinical | Imbioray (Hangzhou) Biomedicine Co Ltd | Solid tumours; Neoplasms | Details |
| CBA-1535 | CBA-1535 | Phase 1 Clinical | Chiome Bioscience Inc | Small Cell Lung Carcinoma; Triple Negative Breast Neoplasms; Mesothelioma; Carcinoma, Non-Small-Cell Lung | Details |
| Anti-5T4 CAR-raNK Cell Therapy | Phase 1 Clinical | Imbioray (Hangzhou) Biomedicine Co Ltd | Solid tumours | Details | |
| Prostate cancer vaccine(inactivated viral)(University of Oxford) | Clinical | University Of Oxford | Prostatic Neoplasms | Details |
This web search service is supported by Google Inc.
