 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> Transition to GMP

Raw and Ancillary Materials Selection Strategy

The success of cell therapy products, including CAR-T cell therapy, is significantly dependent upon the process and material-selection strategies set in the early stages of development. To facilitate the translation into clinical trials, an ancillary (i.e. raw) material selection strategy that considers the end goal is critical. Most long-term strategies emphasize the use of the highest grade of raw materials available as early as possible; however, this may not always be feasible. Accounting for material grade transitions can help balance both performance and costs when moving onto the next stage. Since these raw materials have a significant impact on both the quality and safety of the final products, it is also important to source them from companies that can provide them at a standard that adheres to both local and international regulations.
We understand the complexity of developing an appropriate raw material selection strategy. Regardless of where you are in your cell therapy development, you can rely on us to provide high-quality and cost-effective materials of the appropriate grade that fit your process.

Why consider our GMP products?

GMP products are manufactured under a stringent quality management system that monitors the production process and quality control, while providing a comprehensive set of documentation. To support our customers with high-quality and guaranteed safe GMP products, we are fully committed to upholding the guidelines in GMP manufacturing. We extensively evaluate our products for use as ancillary materials in ex vivo manufacturing including comprehensive viral residue testing and animal in vivo safety experiments. Our strict adherence to GMP helps us bolster your IND application without disrupting your development process.
Identifying a balance between requirements, performance, and costs while seeking qualified, reliable, and consistent raw material manufacturers are all part of the material-selection strategy that is critical in developing a cell therapy product. Early-stage incorporation of higher-grade materials (i.e. GMP) early in the development process can simplify the transition into clinical stages. However, this does not mean GMP grade materials are the only option. ACROBiosystems offers a diverse number of raw material products in several grades that support cell therapy manufacturers throughout the entire development process. Our premium-grade products offer similar bioactivity and performance at a fraction of the price, ideal for preclinical applications. When you are ready to transition into the clinical phase, our premium grade products can be seamlessly transitioned into our GMP grade materials.

| Premium Grade | GMP Grade | |
|---|---|---|
| Application | Research and Development; Preclinical research and transition into clinical phases. | Designed to meet clinical phase requirements and bolster your IND application to various regulatory bodies. v |
| Quality System | ISO 9001 and 13485 Quality Management System | ISO 9001 and 13485 Quality Management System (R&D stage) including GMP Quality Management System (Production stage) |
| Production | ISO certified Workshop | GMP grade workshop certified by third-party audits |
| Transient, stable cell lines | Stable cell lines (Comprehensive external inspections) | |
| Mostly animal-origin free materials | Animal-origin free materials | |
| Pharmaceutical-grade materials | Pharmaceutical-grade materials | |
| Strict secondary sterilization filtration | Strict secondary sterilization filtration | |
| Laminar flow cleanroom with manual fill finish | B+A grade cleanrooms with automated fill finish | |
| No specific virus removal or inactivation process | Specific virus removal / inactivation process (nanofiltration + low pH) | |
| Quality Control | Sterility / Mycoplasma testing | Sterility / Mycoplasma testing |
| Endotoxin control and detection | Endotoxin control and detection | |
| Validated key production equipment and analytical instruments | Strict verification, auditing, and tracking of all equipment and methods. | |
| Process-related impurity testing (DNA, HCP, Residue) | Process-related impurity testing (DNA, HCP, Residue) | |
| No additional quality control tests | Comprehensive virus residue testing, animal in vivo safety experiments | |
| Documentation | Minimum documentation and certifications | Comprehensive regulatory support documentation |
| DMF files (Few products) | DMF files (All products) |

| GMP Grade | |
|---|---|
| Application | Designed to meet clinical phase requirements and bolster your IND application to various regulatory bodies. v |
| Quality System | ISO 9001 and 13485 Quality Management System (R&D stage) including GMP Quality Management System (Production stage) |
| Production | GMP grade workshop certified by third-party audits |
| Stable cell lines (Comprehensive external inspections) | |
| Animal-origin free materials | |
| Pharmaceutical-grade materials | |
| Strict secondary sterilization filtration | |
| B+A grade cleanrooms with automated fill finish | |
| Specific virus removal / inactivation process (nanofiltration + low pH) | |
| Quality Control | Sterility / Mycoplasma testing |
| Endotoxin control and detection | |
| Strict verification, auditing, and tracking of all equipment and methods. | |
| Process-related impurity testing (DNA, HCP, Residue) | |
| Comprehensive virus residue testing, animal in vivo safety experiments | |
| Documentation | Comprehensive regulatory support documentation |
| DMF files (All products) |

| Premium Grade | |
|---|---|
| Application | Research and Development; Preclinical research and transition into clinical phases. |
| Quality System | ISO 9001 and 13485 Quality Management System |
| Production | ISO certified Workshop |
| Transient, stable cell lines | |
| Mostly animal-origin free materials | |
| Pharmaceutical-grade materials | |
| Strict secondary sterilization filtration | |
| Laminar flow cleanroom with manual fill finish | |
| No specific virus removal or inactivation process | |
| Quality Control | Sterility / Mycoplasma testing |
| Endotoxin control and detection | |
| Validated key production equipment and analytical instruments | |
| Process-related impurity testing (DNA, HCP, Residue) | |
| No additional quality control tests | |
| Documentation | Minimum documentation and certifications |
| DMF files (Few products) |
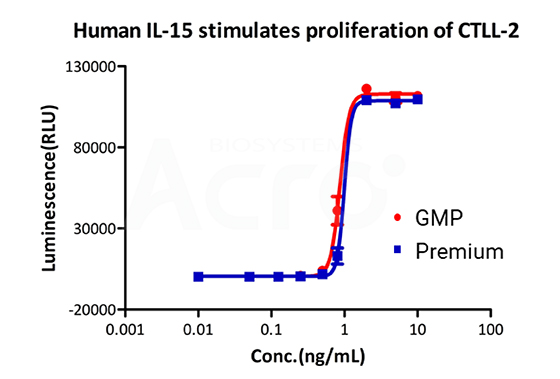
Recombinant Human IL-15 Protein, premium grade (IL5-H4117) designed for preclinical stage use, has the same activity and performance with GMP-grade IL-15 (GMP-L15H13), which enables a seamless transition from preclinical devleopment to clinical phases.
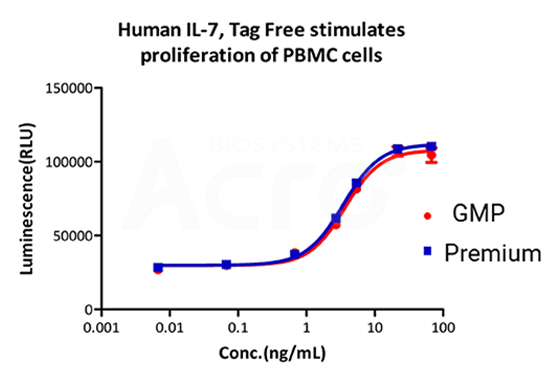
Recombinant Human IL-7 Protein, premium grade (IL7-H4219) designed for preclinical stage use, has the same activity and performance with GMP-grade IL-15 (GMP-L0724), which enables a seamless transition from preclinical devleopment to clinical phases.
Transitioning to GMP
Learn how ACROBiosystems’ products eases the transition from RUO to GMP materials
Regulatory Support Files
Explore the documentation that is available with your ACROBiosystems’ product.

Frequently Asked Questions
Answers that can help guide you on your journey in developing a new therapeutic product.
Other CGT Resources
Explore our expansive materials on cell and gene therapy (CGT) and learn more.
This web search service is supported by Google Inc.
