Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
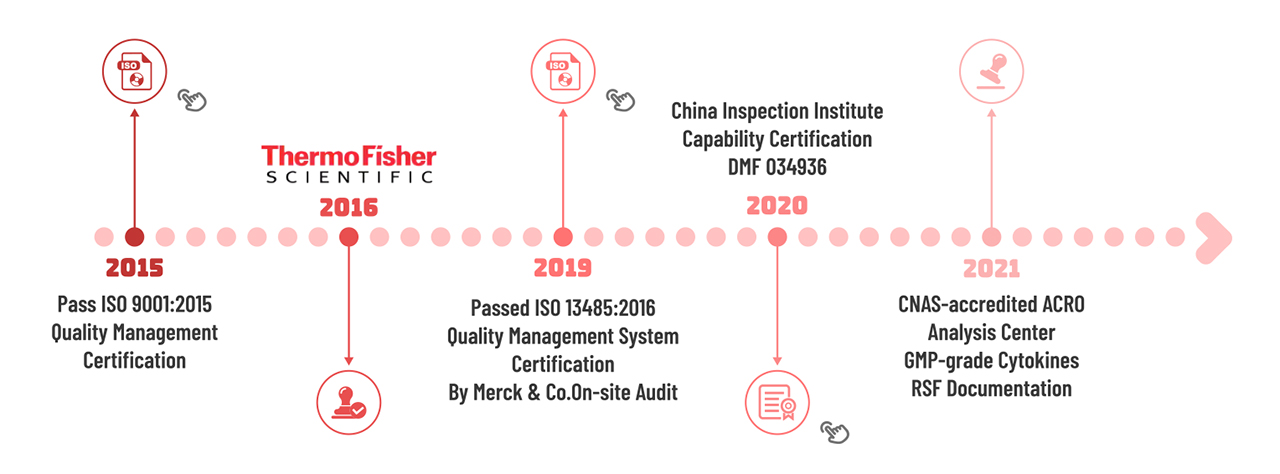
> Quality Management System

The successful translation of your cell therapy products hinges upon material-selection decisions that impact manufacturing. Raw materials chosen in the early stages need to conform to all regulatory criteria when entering clinical trials and later stages. At ACROBiosystems, we ensure that our GMP products follow regulatory standards from internationally recognized bodies including ISO, US and European Pharmacopeias. Furthermore, we take pride in our comprehensive and strict quality management system to produce our products. Especially in cell therapy products where safety matters, we uphold our strict expectations to ensure the ancillary materials we provide to you do not impact your final product.

USP<92>
Growth Factors and Cytokines Used in Cell Therapy Manufacturing

USP<1043>
Ancillary Materials for Cells, Gene, and Tissue-Engineered Products.

Ph. Eur Gen. Chapter 5.2.12
Raw materials of biological origin for the production of cellbased and gene therapy medicinal products.

ISO/TS 20399-2-2018
Growth Factors and Cytokines Used in Cell Therapy Manufacturing
At ACROBiosystems, we are always continuing to improve and manage to our quality management system (QMS) to assist our customers in the development of their cell therapy product. Our GMP quality management system meets and exceeds the regulatory requirements for raw and ancillary materials to assure our customers of our product quality. We welcome our industry partners to conduct on-site audits of our facilities. We are also firmly committed to maintaining the confidentiality of any business cooperations to help further promote collaborations.

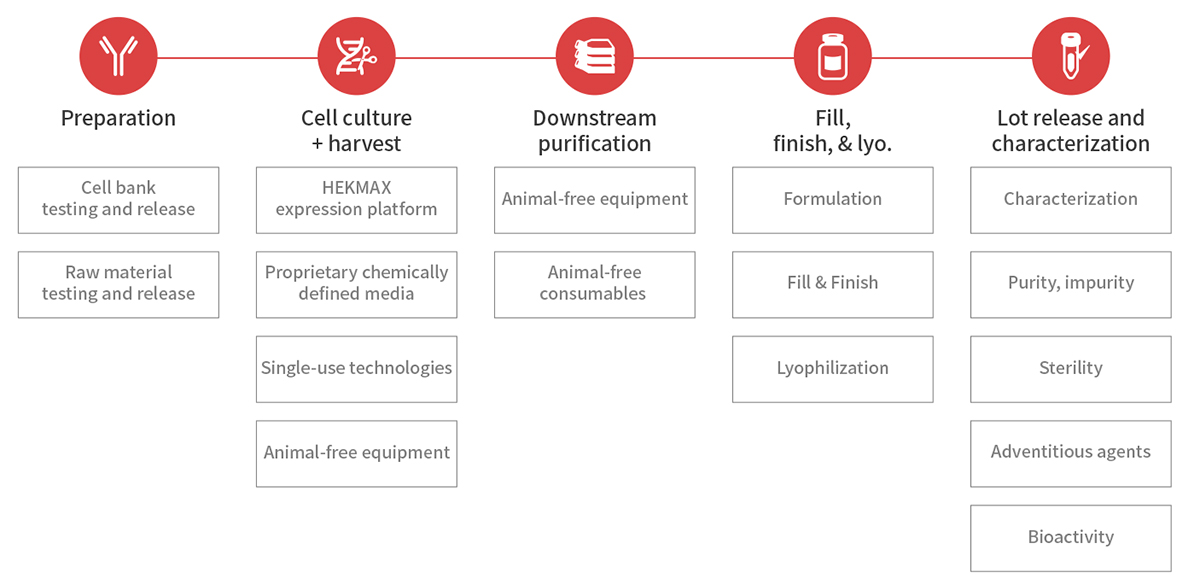
Equipment and Facilities

Our GMP facilities include ISO Level 5 cleanrooms throughout the entire production process. Each step is automated or performed under sterile conditions to minimize any risk of external contamination.
Material Procurement
Our GMP products use pharmaceutical-grade packaging materials to ensure that there is a verified seal quality and safety with no evaporation. Our pharmaceutical-grade glass vials and film-coated rubber stoppers ensure the long-term stability and seal of our final product and slow the penetration of moisture in the air.


Laboratory Quality Control

Our GMP quality control standards are one of the strictest in the industry. Extensive testing including viral, TSE/BSE, mycoplasma, and acute/abnormal toxicity, is performed throughout the production process to ensure that our products do not impact your final product.
![]() DS-PAGE>95%
DS-PAGE>95%
![]() Endotoxin level less than 10 EU/mg
Endotoxin level less than 10 EU/mg
![]() Residual Host Cell DNA content less than 0.02ng/μg
Residual Host Cell DNA content less than 0.02ng/μg
![]() Residual Host Cell Protein content less than 0.5ng/ug
Residual Host Cell Protein content less than 0.5ng/ug
![]() Biological activity >0.8 x 107 IU/mg
Biological activity >0.8 x 107 IU/mg
((Reference the WHO Human IL-15 (NIBSC code: 90/530) as standard))
![]() Mycoplasma testing
Mycoplasma testing
![]() In vitro virus assay
In vitro virus assay
![]() Comprehensive stability data support
Comprehensive stability data support
(accelerated, freeze-thaw, long-term, shipping stability verification)
![]() Batch-to-batch consistency
Batch-to-batch consistency
![]() Microbial testing and more...
Microbial testing and more...

Regulatory Support Files

We understand that full transparency and traceability is critical in developing your cell therapy product. For our GMP grade products, ACROBiosystems offers comprehensive documentation including detailed quality inspection, analytical method verification reports, and many others. Click below to learn more about what documents we offer in our Regulatory Support File.
Transitioning to GMP
Learn how ACROBiosystems’ products eases the transition from RUO to GMP materials
Regulatory Support Files
Explore the documentation that is available with your ACROBiosystems’ product.

Frequently Asked Questions
Answers that can help guide you on your journey in developing a new therapeutic product.
Other CGT Resources
Explore our expansive materials on cell and gene therapy (CGT) and learn more.
This web search service is supported by Google Inc.