Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
> GMP-grade Proteins ACROBiosystems’ high-quality GMP proteins offer the safety, consistency, and efficacy to support the development of your cell therapy product.

![]() GMP-grade Proteins for your Needs
GMP-grade Proteins for your Needs
ACROBiosystems produces our GMP proteins in large clinical trial size lots. Secure your material supply and improve cell quality consistency.
![]() Excellent Safety Profile
Excellent Safety Profile
Our GMP quality standards are one of the strictest in the industry. Sterility, mycoplasma, endotoxin, and residual impurities are tested throughout the production process to ensure that our cytokines do not impact your final product.
![]() Consistent Bioactivity for Manufacturing
Consistent Bioactivity for Manufacturing
Our strict control and quality management systems help us ensure quality consistency between our GMP cytokine batches. You can be confident in using ACROBiosystems’ animal-origin free GMP cytokines for clinical phases with your ground-breaking therapies.
![]() Bolster your IND Application
Bolster your IND Application
Our GMP grade products have been extensively evaluated for use as ancillary materials in ex vivo cell manufacturing. Our strict adherence to GMP helps support your IND application without disrupting your development process.

| Molecule | Cat. No. | Product Description | Preorder/Order |
|---|---|---|---|
| IL-15 | GMP-L15H13 | GMP Human IL-15 | |
| IL-7 | GMP-L07H24 | GMP Human IL-7 | |
| IL-21 | GMP-L21H25 | GMP Human IL-21 |
ACROBiosystems GMP Quality Management System
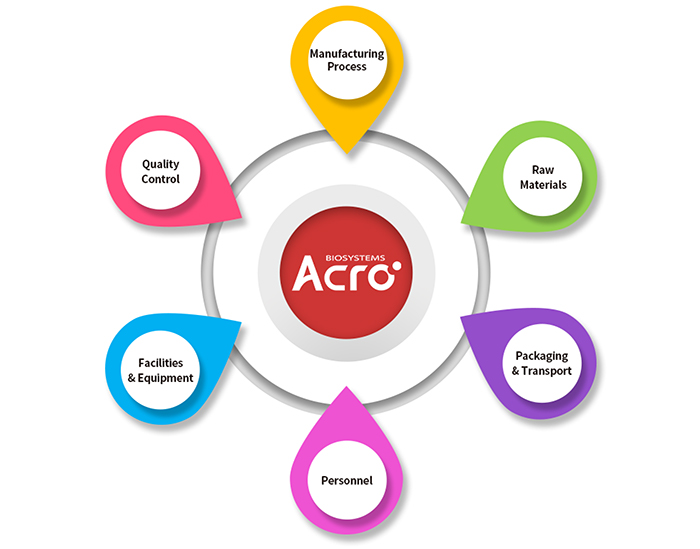
Especially in the pre-clinical and clinical phases, we understand that the safety and quality of raw and ancillary materials are of the utmost importance. Our recombinant proteins are manufactured and tested in accordance to the relevant USFDA GMP (Good Manufacutring Practice) guidelines under an ISO 9001 and ISO 13485 quality management system with ani- mal-origin free (AOF) materials. To ensure our products do not impact your final product, we take a step further by implementing stricter quality controls that include mycoplasma, viral, and in vivo toxicity tests.

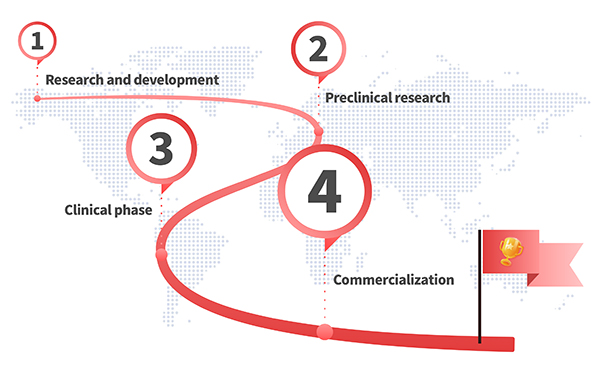
Custom GMP Protein Services

We are with you every step of the way. Each and every one of our custom products are developed based on your needs starting from your pilot research application to large-scale production. No matter what stage your research is in, let us know what you need and we will connect you with our experts to assist you.
Transitioning to GMP
Learn how ACROBiosystems’ products eases the transition from RUO to GMP materials
Regulatory Support Files
Explore the documentation that is available with your ACROBiosystems’ product.

Frequently Asked Questions
Answers that can help guide you on your journey in developing a new therapeutic product.
Other CGT Resources
Explore our expansive materials on cell and gene therapy (CGT) and learn more.
This web search service is supported by Google Inc.