Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>


































 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
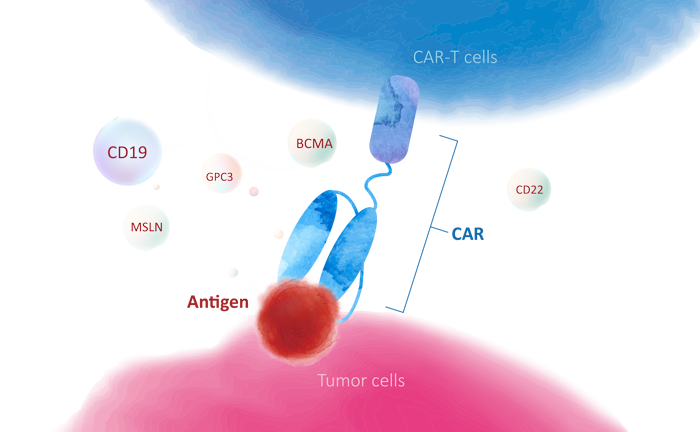
> CD19:a validated therapeutic target for B-cell malignancies 
CD19 is a B-cell surface protein expressed throughout B-cell development and it is expressed on nearly all B-cell malignancies, including chronic lymphocytic leukemia (CLL), ALL, and many non-Hodgkin lymphomas. This specificity and universal expression for a single cell lineage has made CD19 an attractive target for antibody-based therapy, including bispecific antibodies, ADCs, Fc-engineered antibodies and CAR-T cell therapy. So far, two anti-CD19 CAR-T cell therapies and one bispecific anti-CD19/CD3 antibody have been approved by the FDA to treat hematologic B-cell malignancies. Till May 7, 2020, according to ClinicalTrials.gov, more than 763 clinical trials have been initiated.
To support the development of CD19-targeted antibody-based therapy, ACROBiosystems has developed a comprehensive series of CD19 protein products, including unconjugated CD19, biotinylated CD19 and fluorescent-labeled CD19. These proteins are suitable for immunization, antibody screening, antibody affinity measurement and detection of anti-CD19 CAR expression. The high activity of these proteins has been verified by ELISA, SPR and flow cytometry (FACS). Protocols are offered for free, which can help you save the development cycle.
ACROBiosystems has submitted DMF for its recombinant CD19 to FDA, and filed the DMF number as 034936. If our DMF filed CD19 proteins have been used in your drug development process, you can request that we provide DMF authorization to FDA in support of a submission or filing that you have made to the FDA. Please submit your request to ACROBiosystems by leaving your information here.Request for Authorization
| Molecule | Cat. No. | Host | Product Description | Structure |
|---|
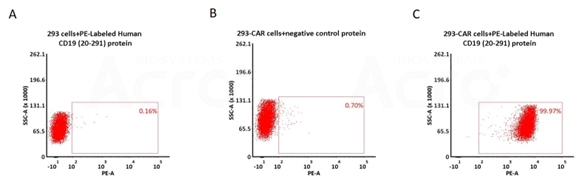
5e5 of anti-CD19 CAR-293 cells were stained with 100 μL of 1:50 dilution (2 μL stock solution in 100 μL FACS buffer) of PE-Labeled Human CD19 (20-291), His Tag (Cat. No. CD9-HP2H5) and negative control protein respectively (Fig. C and B), and non-transfected 293 cells were used as a control (Fig. A). PE signal was used to evaluate the binding activity (QC tested).
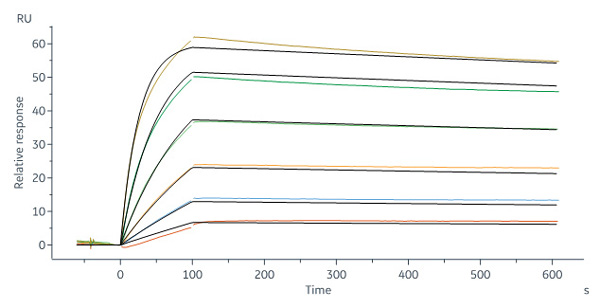
Biotinylated Human CD19 (20-291), His, Avitag (Cat. No. CD9-H82E9) captured on Biotin CAP - Series S sensor Chip can bind FMC63 MAb (mouse lgG2a) with an affinity constant of 0.255 nM as determined in a SPR assay (Biacore 8K) (QC tested).
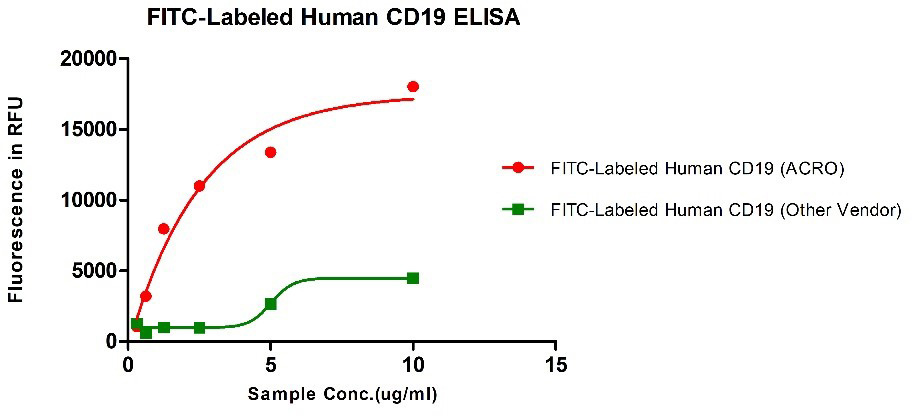
Binding activity of FITC-Labeled Human CD19, His Tag from two different vendors were evaluated in the ELISA analysis against FMC63 Mab. The result showed that ACRO's FITC-Labeled Human CD19, His Tag has a much higher binding activity than that of the other vendor.
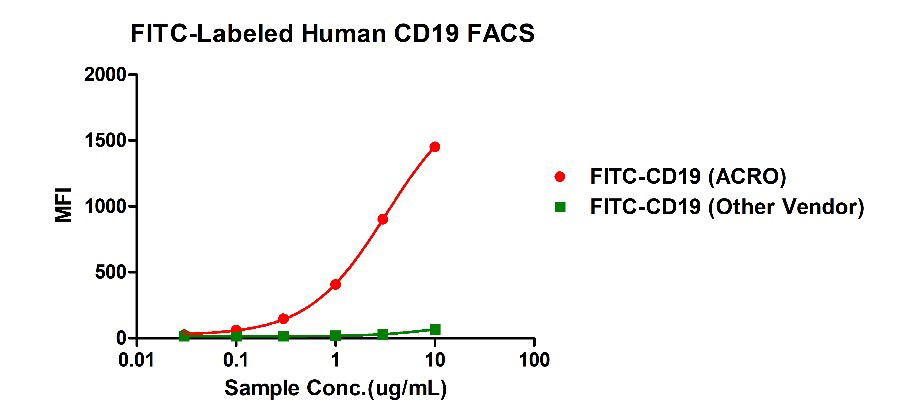
Binding activity of FITC-Labeled Human CD19, His Tag from two different vendors were evaluated in the flow cytometry analysis against anti-CD19-CAR-293 cells. The result showed that ACRO's FITC-Labeled Human CD19, His Tag has a much higher binding activity than that of the other vendor.
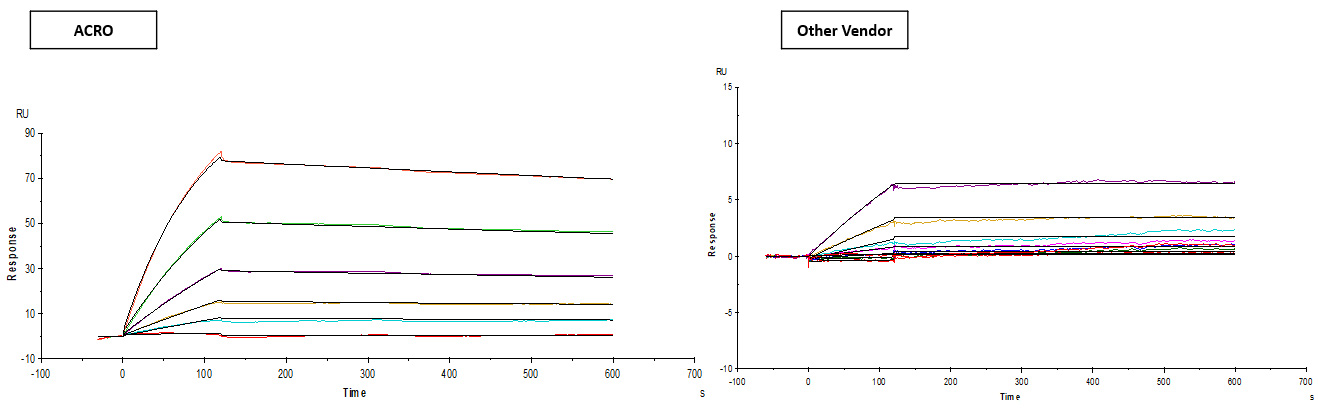
Binding activity of Human CD19, His Tag from two different vendors were evaluated by SPR assay against FMC63 MAb. The result showed that ACRO's Human CD19, His Tag can bind FMC63 MAb with an affinity constant of 2.95 nM which is much higher than that of the other vendor.
This web search service is supported by Google Inc.