 Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit! Request a FREE Sample of our FcRn Binding Kit!
Request a FREE Sample of our FcRn Binding Kit!
 Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.  Limited Edition Golden Llama is here! Check out how you can get one.
Limited Edition Golden Llama is here! Check out how you can get one.
 Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!  Request a FREE sample of our GMP products!
Request a FREE sample of our GMP products!
T cell activation plays a central role in the immune response. Its activation, proliferation and differentiation require dual stimulation signals. The first signal is the combination of TCR/CD3 and antigen-presenting cell (APCs) surface-specific MHC molecule antigen-peptide complexes. The second signal is a non-specific co-stimulatory signal, which is generated by the interaction of multiple pairs of co-stimulatory molecules on the surface of APCs and the corresponding receptors of T cells (such as: CD28, CTLA-4 and CD80, CD86, 4-1BB and 4-1BBL, CD40 and CD40L, PD-1 and PD-L1, etc.), among which CD28 is the most important costimulatory molecule, and the second signal can fully activate T cells, secrete cytokines and expresses cytokine receptors. Studies have shown that the in vitro activation and proliferation of T cells plays an important role in research fields such as cell therapy and immune checkpoints.
CAR-T and TCR-T therapy are the current research hotspots of adoptive immune cell therapy. Whether it is CAR-T or TCR-T cell culture in vitro, T cells need to be activated before subsequent operations can be performed. Therefore, a suitable T cell activation reagent is an essential raw material for the development of cell therapy drugs. At present, the main methods of activation of T cells in vitro on the market are: ① Use soluble antibodies, such as Anti CD3/CD28 antibodies, combined with cytokines such as IL-2 for stimulation; ② Using antibodies bound to solid-phase carriers, such as CD3/CD28 antibody-coupled magnetic beads, and the appropriate T cell activation method can be selected based on the activation strength and subsequent residual detection considerations.
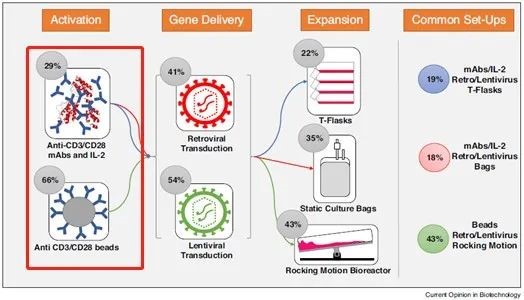
Figure 1 Activation of T cells during cell therapy development
Immune checkpoint drug have become the focus of attention in tumor immunotherapy due to their excellent clinical efficacy, particularly antibody targeting immune checkpoints, a hot spot in immunotherapy, have become the forefront of competition among pharmaceutical giants. The key points of antibody development are not only functional effects, but activity assays in vitro are also important, which can provide early data support for functional efficacy in vivo. At present, the in vitro activity research methods for immune checkpoint antibody mainly focus on the activation activity of T cells. For example, CD3 antibodies can be coated on PBMC culture plates, and CD28 soluble antibodies can be added to the supernatant to activate T cells, or by CD3 /CD28 antibody coupled with magnetic beads to activate, and then the content of cytokines in the culture supernatant containing the immune checkpoint drug was detected to reflect the activation of the drug on T cells.
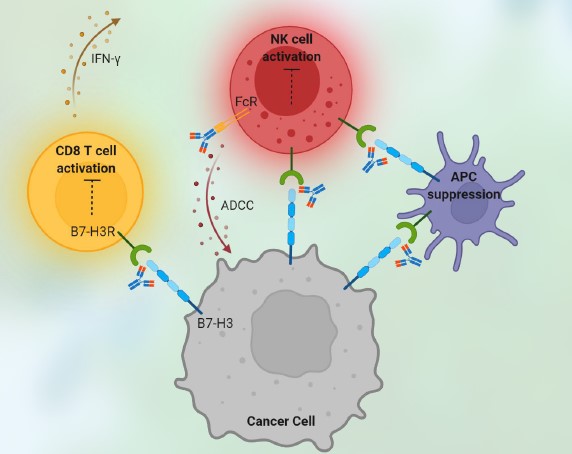
Figure 2 Immune checkpoint drugs killing tumor cells
![]() 5.5 μm size, can better simulate APC and stimulate T cells
5.5 μm size, can better simulate APC and stimulate T cells
![]() Strong magnetism, easy separation, magnetic beads are not easy to remain
Strong magnetism, easy separation, magnetic beads are not easy to remain
![]() Ultra low endotoxin (< 2EU/mg), no damage to T cells
Ultra low endotoxin (< 2EU/mg), no damage to T cells
![]() Verified by cell-based assay, it can efficiently activate and expand T cells
Verified by cell-based assay, it can efficiently activate and expand T cells
![]() Low matrix interference tested by cells culture medium and real samples of serum
Low matrix interference tested by cells culture medium and real samples of serum
![]() Kit standards are calibrated by WHO standard NIBSC/WHO (87/586)
Kit standards are calibrated by WHO standard NIBSC/WHO (87/586)
![]() Both RUO and ClinMax version are available to meet the needs of R&D and clinical trials
Both RUO and ClinMax version are available to meet the needs of R&D and clinical trials
![]() Strict control of sensitivity, specificity, accuracy, linearity and inter-/intra-assay precision
Strict control of sensitivity, specificity, accuracy, linearity and inter-/intra-assay precision
![]() High purity verified by MALS or HPLC
High purity verified by MALS or HPLC
![]() High bioactivity verified by ELISA/SPR/FACS, etc.
High bioactivity verified by ELISA/SPR/FACS, etc.
![]() High batch to batch consistency by strict QC tested
High batch to batch consistency by strict QC tested
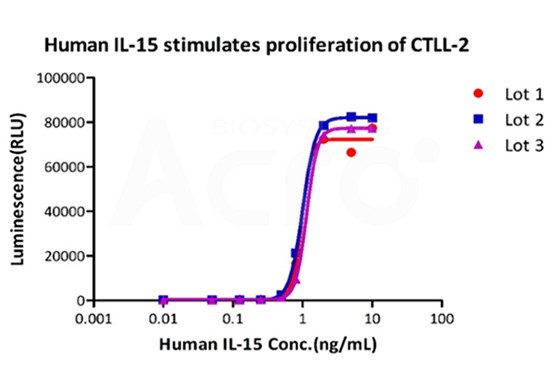
T cells are fully activated by the first and second signal stimulation, and they also depend on a variety of cytokines (IL-1, IL-2, IL-4, IL-6, IL-10, IL-12, IL-15 and IFN-γ, etc.) for further proliferate and activate. Otherwise, activated T cells cannot proliferate and differentiate, resulting in apoptosis. Therefore, media supplements such as IL-15, IL-7, and IL-21 and other cytokines are important reagents for the proliferation and differentiation of T\NK immune cells. Both FDA and CDE have relevant regulations on the use of these key materials. The FDA, CMC recommends using FDA-approved or clinical-grade materials. For Chinese Pharmacopoeia regulations, priority should be given to the use of low-risk materials such as GMP grade materials as opposed to non-GMP grade materials. Therefore, safe, effective, and compliant cytokines are crucial for the success of R&D processes and applications of immune cell therapy drugs.
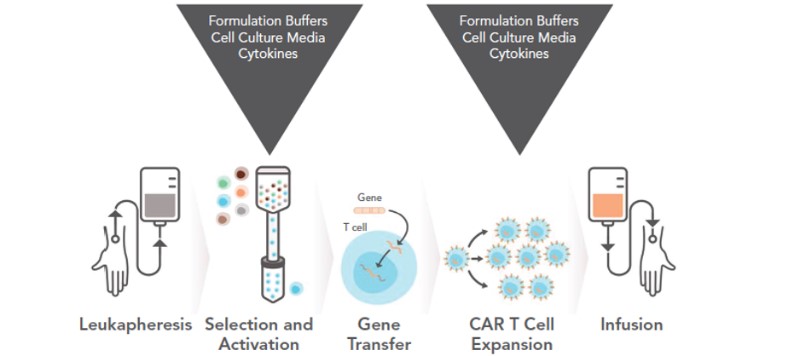
Figure 3 The development of CAR-T requires cytokines and other culture materials
![]() Strict quality control standards
Strict quality control standards
![]() Excellent safety profile (tested for sterility, mycoplasma, endotoxin, and residual impurities)
Excellent safety profile (tested for sterility, mycoplasma, endotoxin, and residual impurities)
![]() High stability and batch-to-batch consistency
High stability and batch-to-batch consistency
![]() Comprehensive regulatory support files
Comprehensive regulatory support files
As an important immune cell, T cells play a key role in the development of immune cell therapy culture. Faced with a variety of T cell culture-related reagents on the market, selecting safe and compliant products is crucial for drug development and regulatory approval. To meet market requirements, ACROBiosystems has developed a series of related products for T cell activation and culture that have been released under strict quality standards to accelerate drug development process.
| Product | Size | Amount |
|---|---|---|
| Human T cell Activation/Expansion CD3/CD28 Beads, premium grade DMF Filed | 2.5 mg | 2.5 × 107 beads |
| 10 mg | 1 × 108 beads |
| Molecule | Cat.No. | Product description | Preorder/Order |
|---|---|---|---|
| IL-15 | GMP-L15H13 | GMP Human IL-15 | |
| IL-7 | GMP-L07H24 | GMP Human IL-7 | |
| IL-21 | GMP-L21H25 | GMP Human IL-21 |
| Molecule | Cat. No. | Product Description | Preorder/Order |
|---|---|---|---|
| IL-2 | CRS-A003 | Human Interleukin-2(IL-2) ELISA Kit coming soon | |
| IL-4 | CRS-A004 | Human Interleukin-4(IL-4) ELISA Kit coming soon | |
| IL-6 | CRS-A005 | Human Interleukin-6(IL-6) ELISA Kit coming soon |
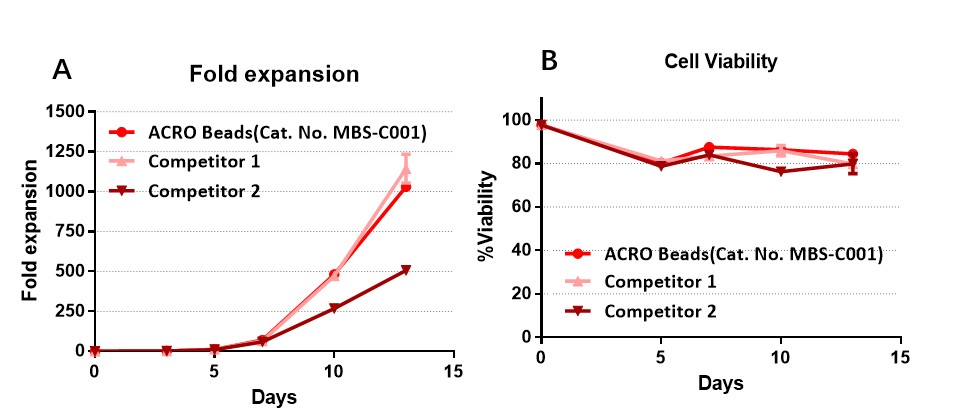
The purified human T cells were stimulated using Human T cell Activation/Expansion CD3/CD28 Beads, premium grade DMF Filed(Cat. No. MBS-C001) and competitor’s beads respectively. Cells were expanded in T cell culture medium supplemented with 4ng/mL of rhIL-2 Protein (Acrobiosystems, Cat. No. IL2-H4113). Activated Cells were expanded for up to 13 days (A) with high cell viability (B).
[1] Kay, J.E., 1991. Mechanisms of T lymphocyteactivation. Immunology Letters 29, 51 – 54
[2] Trickett A, Kwan YL. T cellstimulation and expansion using anti-CD3/CD28 beads. J Immunol Methods. 2003Apr 1;275(1-2):251-5.
[3] Kalos M, Levine BL, Porter DL, KatzS, Grupp SA, Bagg A, June CH: T cells with chimeric antigen receptors havepotent antitumor effects and can establish memory in patients with advancedleukemia. Sci Transl Med 2011, 3:95ra73.
[4] Hollyman D, Stefanski J, PrzybylowskiM, Bartido S, Borquez- Ojeada O, Taylor C, Yeh R, Capacio V, Olszewska M, HoseyJ et al.: Manufacturing validation of biologically functional T cells targetedto CD19 antigen for autologous adoptive cell therapy. J Immunother 2009, 32:169-180.
[5] https://www.chromatographyonline.com/view/physicochemical-methods-for-vectors-and-ancillary-materials-in-cellular-and-gene-therapies
This web search service is supported by Google Inc.
