Leave message
Can’t find what you’re looking for?
Fill out this form to inquire about our custom protein services!
Inquire about our Custom Services >>

































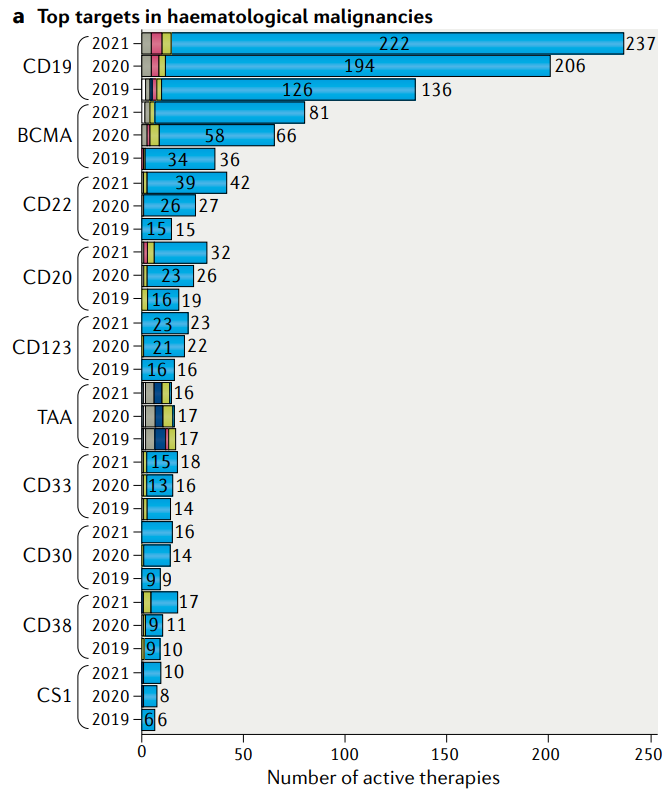
Till April, 2021, according to the data published on ClinicalTrials.gov, there are 1,358 active cell therapy trials, among which most trials are focusing on haematological malignancies, Typical targets include CD19, BCMA and CD22.
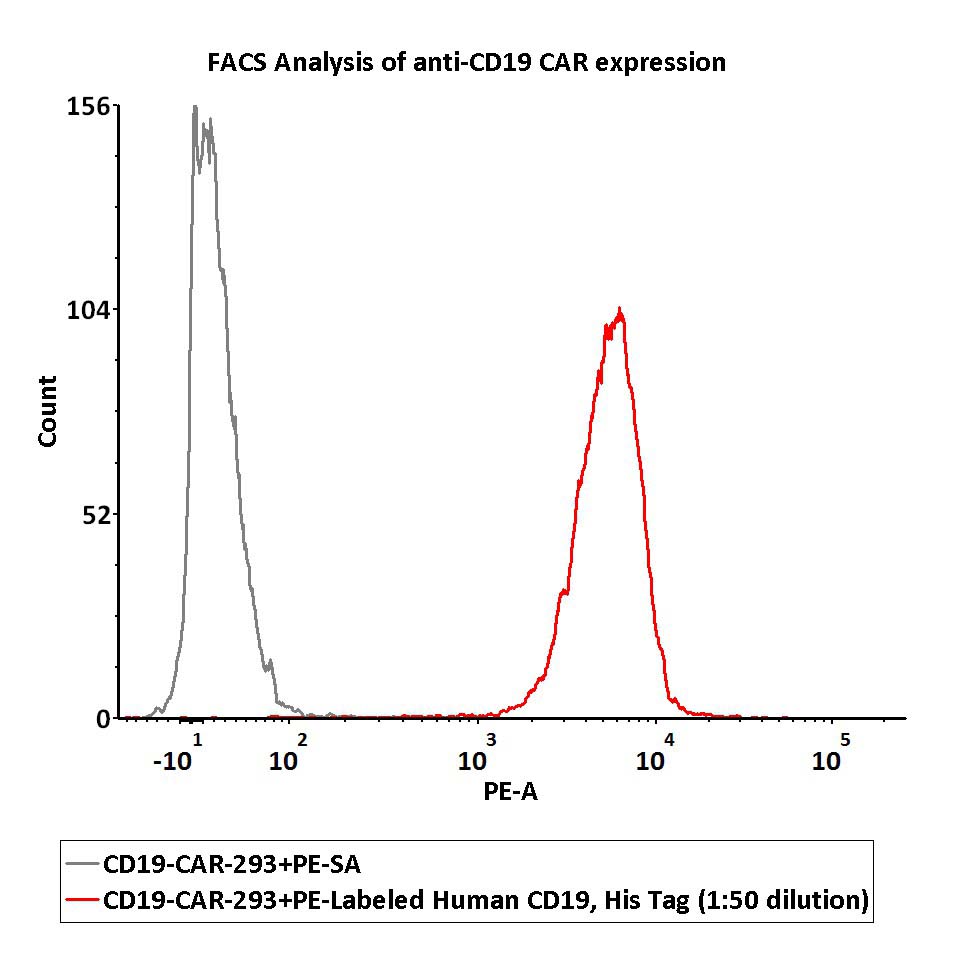
ACROBiosystems offers a series of CAR-T target proteins for haematological tumor, such as CD19, BCMA, CD20, CD30 products, which are suitable for detecting CAR expression in clinical trials.
Cat. No. SI2-HF2H6
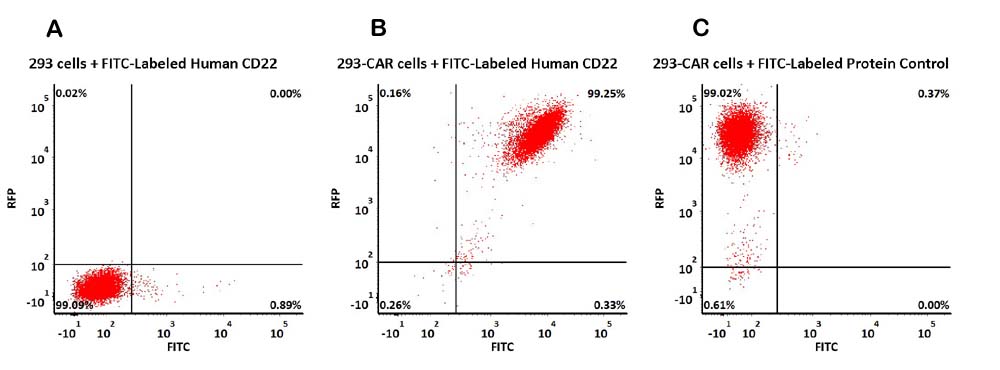
More CAR-T related products
> Click here to learn more about blood tumor targets
> Click here to learn more about Fluorescent-labeled proteins
This web search service is supported by Google Inc.