> Factores de crecimiento para el cultivo de células Para apoyar a los fabricantes y desarrolladores de terapia celular en curso, ACROBiosystems ha desarrollado una amplia gama de citoquinas de alta calidad para el cultivo in vitro de células inmunitarias, células madre, organoides y otros tipos de células más. También ofrecemos grados Premium(Pre-GMP) y GMP para nuestras citoquinas, con el fin de satisfacer mejor las necesidades de las distintas etapas de desarrollo de fármacos y aplicaciones. Los tres grados de citoquinas se fabrican mediante un proceso de producción similar, lo que permite una transición sin problemas entre los grados. Como resultado, le permitimos una transición fluida entre nuestros productos y aceleramos su investigación y desarrollo.
| Grado Premium(Pre-GMP) | Grado GMP | |
|---|---|---|
| Application | Research and Development; Preclinical research, seamless transition into clinical phases | Designed to meet clinical phase requirements and bolster your IND application to various regulatory bodies. |
| Quality System | ISO 9001 /ISO 13485 Certified | ISO 9001 /ISO 13485 Certified (Development stage) GMP Quality Management System (Production stage) |
| Production | ISO certified facilities | GMP certified facilities |
| Transient or stable cell lines | Stable cell lines (Full inspection according to USP and ChP) | |
| Animal-origin free materials or BSE/TSE free | Animal-origin free materials or BSE/TSE free | |
| Pharmaceutical-grade materials | Pharmaceutical-grade materials | |
| Strict sterilization filtration | Strict sterilization filtration | |
| Class C+A room with manual aseptic filling (ISO5) | B+A Cleanroom aseptic filling | |
| No additional virus clearance steps | Additional specific virus removal processes | |
| Quality Control | Sterility / Mycoplasma testing | Sterility / Mycoplasma testing |
| Endotoxin control and detection | Endotoxin control and detection | |
| Validated key equipment and analytical instruments | Validated equipment /analytical instruments/analytical methods(Audit trail available) | |
| Residual DNA/HCP testing | Residual DNA/HCP testing | |
| Limited adventitious agent testing | Stricter standards for viral residues alongside animal safety evaluations (select products). | |
| Documentation | Common regulatory support | Comprehensive regulatory support files |
| DMF filing (Few products) | DMF filing (All products) |
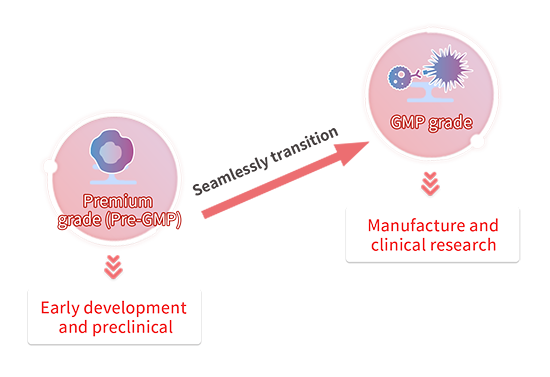
Transición de Premium(Pre-GMP) a GMP 
Las citoquinas son materias primas fundamentales utilizadas en el cultivo de células para los medicamentos de terapia celular y génica (CGT). Por lo general, en la fase preclínica no se prioriza ni la seguridad ni la calidad de las materias primas. En este caso, se pueden utilizar productos de uso exclusivo para la investigación. Sin embargo, cuando se pasa a fases posteriores de desarrollo de fármacos, como la CMC o la clínica, es necesario sustituir las materias primas por materiales de grado GMP para cumplir con las directrices reglamentarias. En este período de transición, se dedica una gran cantidad de energía a reevaluar y validar nuevas materias primas.
Para facilitar esta transición del desarrollo preclínico a la etapa clínica, ofrecemos varios grados de citoquinas que han sido evaluados para ser casi idénticos en bioactividad, junto con la documentación requerida de los productos GMP. Recomendamos el uso de nuestras materias primas de primera calidad en la fase inicial de desarrollo para pasar sin problemas a nuestras materias primas de grado GMP al entrar en las fases CMC o clínica y minimizar el número de estudios de reevaluación y validación realizados.
To match your project schedule, shorten decision times, and save your research and development budget, we are currently offering a free trial of our non-GMP grade products along with our custom GMP product services!
During this campaign, if you are looking for custom GMP-grade products that are a part of our catalog on the official website of ACROBiosystems (www.acrobiosystems.com), we will provide you with a free trial size of our non-GMP grade products (100 µg) to test. Our non-GMP grade products have the same performance as our GMP-grade products for a sneak-peek into our GMP product performance and assist with your transition into GMP!
![]() Estéril
Estéril
![]() Libre de Origen Animal
Libre de Origen Animal
![]() Alta Pureza≥95%
Alta Pureza≥95%
![]() Alta Bioactividad Verificada por Ensayo Celular
Alta Bioactividad Verificada por Ensayo Celular
![]() grados Premium(Pre-GMP) y GMP disponibles
grados Premium(Pre-GMP) y GMP disponibles
![]() Baja Endotoxina≤0.1 EU/μg
Baja Endotoxina≤0.1 EU/μg
![]() Sin portador
Sin portador
![]() Similar a la conformación y modificaciones naturales
Similar a la conformación y modificaciones naturales
![]() Consistente entre lotes
Consistente entre lotes
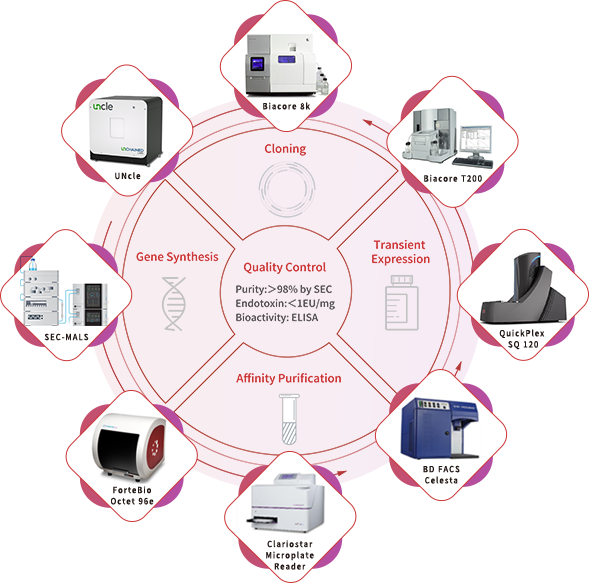
With a portfolio of over 5,000 recombinant proteins and an industry-leading, scale-up ready protein development platform, ACROBiosystems has accumulated over 10 years of experience in developing recombinant proteins. Using this platform, our custom GMP-grade protein services are designed to ensure that our proteins are both structurally designed and validated for cellular therapies manufacturing. We take care to adhere strictly to the GMP guidelines with our comprehensive quality management system and quality controls, providing you with high-quality raw materials without disrupting your development process. Our custom GMP-grade protein service is a one-stop service based on your needs to maximize your therapy’s success. We offer two different developmental processes: converting our non-GMP protein products to GMP or developing a custom GMP-grade protein product from scratch.
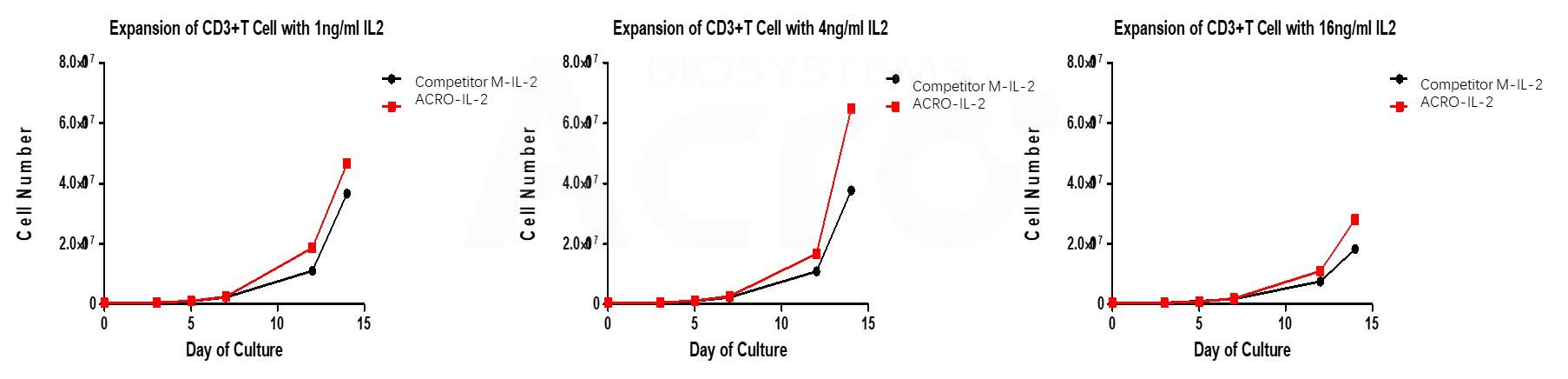
ACRO’s Human IL-2, premium grade (Cat. No.IL2-H5215) has higher bioactivity than that of other competitors when activates T cell with CD3/CD28 magnetic beads at different concentrations.
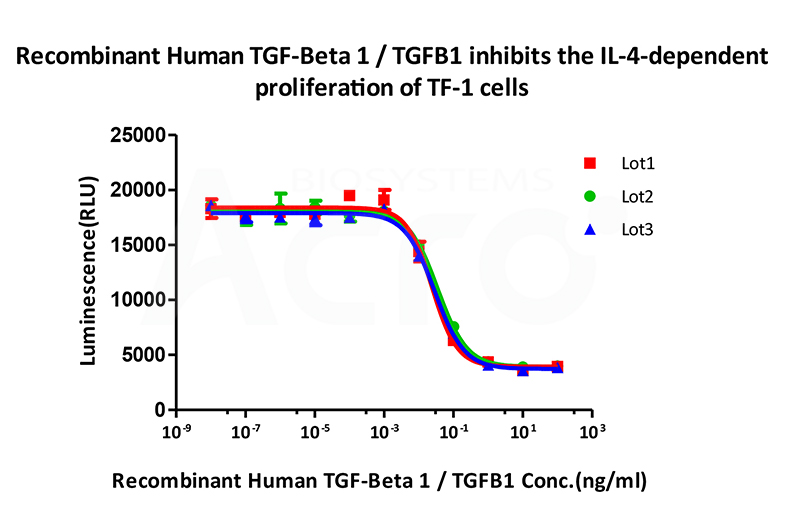
Bioactivity of three different lots of Recombinant Human TGF-Beta 1 TGFB1 (Cat. No. TG1-H4212) is verified by cell-based assay, and the result shows very high batch-to-batch consistency.
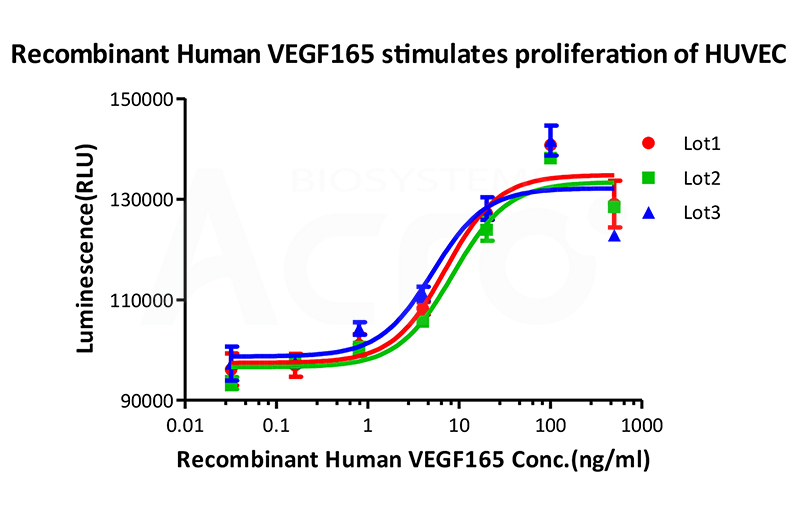
Bioactivity of three different lots of Recombinant Human VEGF165 (Cat. No. VE5-H4210) is verified by cell-based assay, and the result shows very high batch-to-batch consistency.
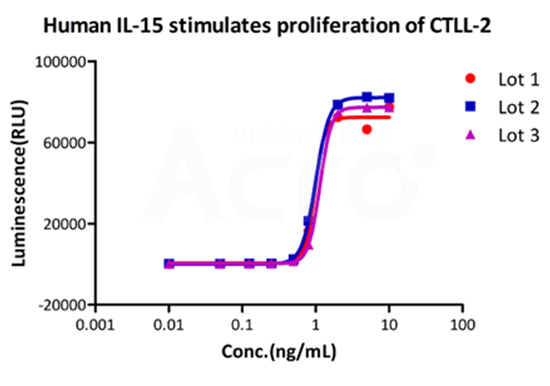
Recombinant Human IL-15 Protein (premium grade), designed for preclinical stage, has the same functional activity and performance as GMP Grade IL-15 (Cat. No.GMP-L15H13), which enables a seamless transition from preclinical development to clinical phases.
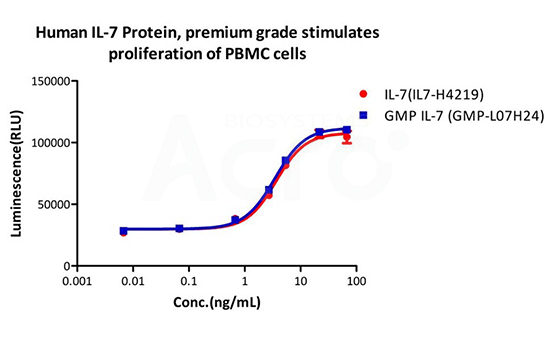
Human IL-7 Protein (premium grade) designed for preclinical stage, has the same functional activity and performance as GMP Grade IL-7 (Cat. No. GMP-L07H24), which enables a seamless transition from preclinical development to clinical phases.
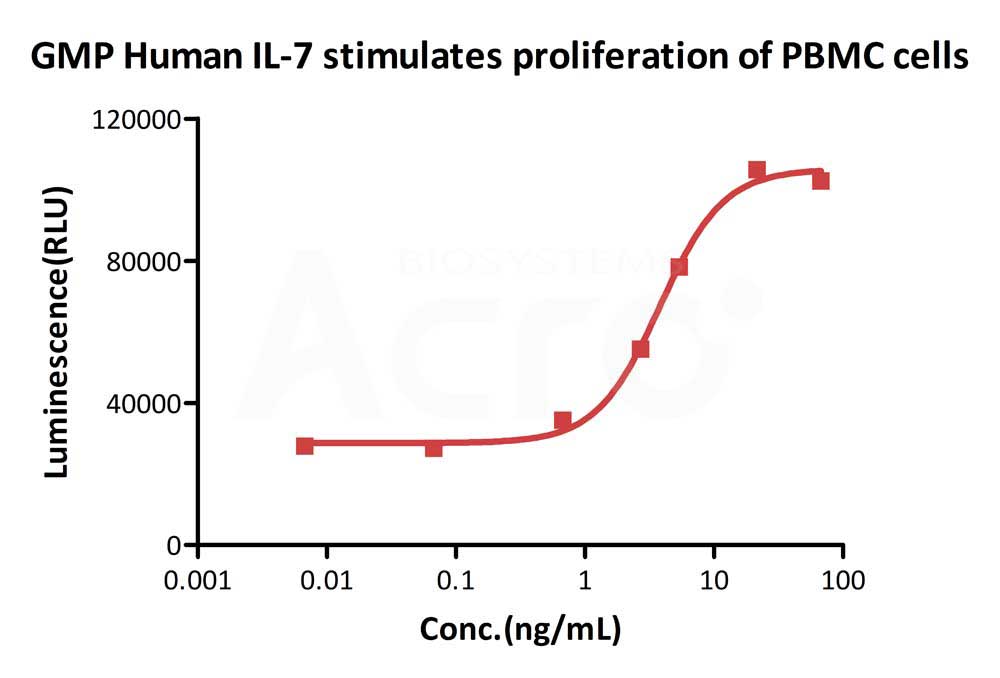
GMP Human IL-7 (Cat. No.GMP-L07H24) stimulates proliferation of PHA-P-activated human peripheral blood mononuclear cell (PBMC). The EC50 for this effect is 3.821 ng/mL, corresponding to a specific activity of > 1.0 ⅹ10^8 IU/mg, which is calibrated against human IL-7 WHO International Standard (NIBSC code: 90/530) (QC tested).
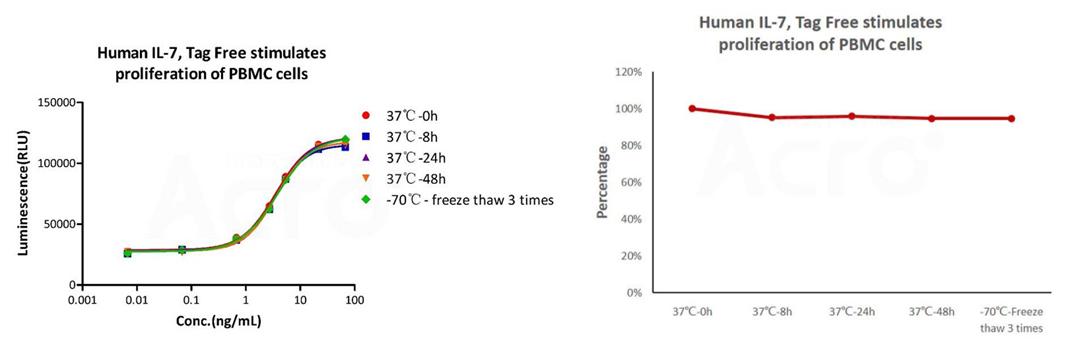
The cell-based assay shows that GMP Human IL-7 (Cat. No. GMP-L07H24) is stable at 37°C for 48 hours and after freezing and thawing 3 times.

Bioactivity of three different lots of GMP Human IL-15 (Cat. No.GMP-L15H13) verified by cell-based assay, and the result shows very high batch-to-batch consistency.
This web search service is supported by Google Inc.
